Paper out: Regulation of Filamin C by Protein Phosphatase-1
11/29/2024A new study performed by Johannes Zimmermann and former lab mate Anja Schwäble from the lab of Bettina Warscheid together with Thomas Kokot from the lab of Maja Köhn investigates the role of protein phosphatase-1 (PP1) in skeletal muscle cells during mechanical stress.
FLNc Regulates Z-Disc Development and Maintenance in Skeletal Muscle Cells
For living organisms, enduring and overcoming various forms of cellular stress while maintaining a functional proteome and homeostasis is a fundamental requirement. Among these stressors, mechanical force has recently been recognized as an important source of acute or chronic cellular stress in multicellular organisms. During physical activity, mechanical strain exerts significant force on cross-striated muscle cells, particularly on the mechanosensitive components of the myofibrillar Z-disc, which defines the boundary of adjacent sarcomeres. Aberrant forms of Z-disc and Z-disc-associated proteins are linked to various muscle diseases, including myopathies and cardiomyopathies, which lead to progressive muscle weakness and heart failure.
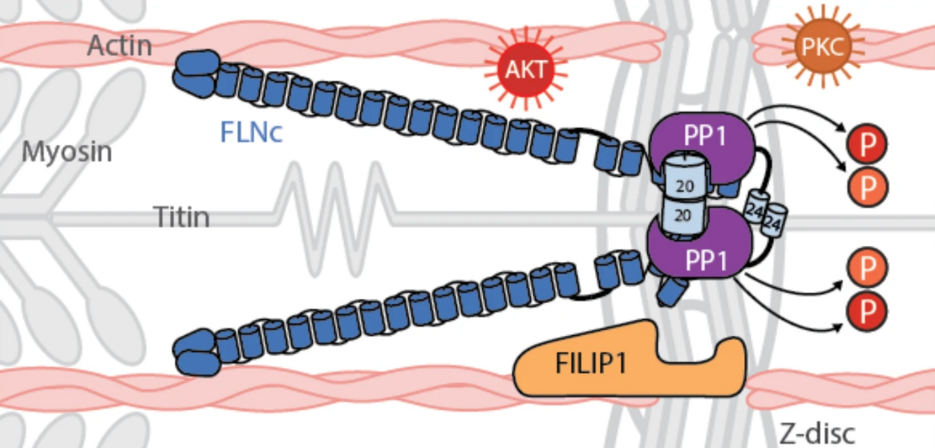
A key component of the myofibrillar Z-disc is the large actin-binding protein filamin C (FLNc). FLNc is predominantly expressed in cross-striated muscle cells, whereas the other two filamin family members, FLNa and FLNb, are more ubiquitously expressed across tissues. Filamins function as structural scaffolds and signaling platforms, supported by their structure, which includes an amino-terminal actin-binding domain followed by 24 immunoglobulin-like (Ig-like) domains (d1-d24). This rod-like structure is augmented by hinge regions providing flexibility, while the carboxy-terminal d24 domain facilitates filamin homodimerization for efficient actin cross-linking. Given its structural attributes and dynamic properties, FLNc is crucial for muscle development, Z-disc assembly, and the maintenance and repair of myofibrils, which are continuously subjected to mechanical strain. Furthermore, FLNc deficiency results in embryonic lethality in mice, and mutations in the FLNC gene are associated with skeletal myopathies and cardiomyopathies in humans.
New insights from Kokot et al.
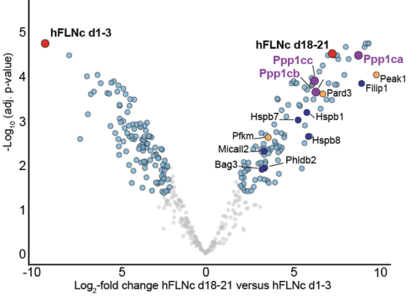
Under mechanical stress, mFLNc-S2234 phosphorylation decreases despite elevated AKT activity, which suggests a dominant role for phosphatase activity in regulating this site. To explore this further, affinity purification-mass spectrometry (AP-MS, see figure below) and in vitro assays were conducted, identifying protein phosphatase 1 (PP1) as a key regulator. The unbiased AP-MS analysis played a central role in the initial the initial determination of possible interactors of FLNc, since it the necessary sensitivity to find low abundant interactors, as well as inteactors, as well as offering the possibility of analyzing all proteins of the human proteome in this analysis. PP1 was shown to efficiently dephosphorylate FLNc-pS2234, thereby facilitating FILIP1 binding. These findings underscore PP1 as a critical mediator in the reversible regulation of FLNc during mechanical stress.
In addition, FILIP1 binding to FLNc was found to be phosphorylation-dependent and directly linked to FLNc degradation. Building on this, the study demonstrates that PP1c-mediated dephosphorylation of FLNc at S2233/S2234 significantly enhances its interaction with FILIP1 in skeletal myocytes. Specifically, pulldown assays confirmed an increase in binding of both recombinant and endogenous FLNc to FILIP1 following PP1c treatment, thereby highlighting a reversible regulatory mechanism governed by FLNc dephosphorylation.
Taken together, our data show that PP1 efficiently dephosphorylates mFLNc-pS2234 in C2 myotubes. Through this action, PP1 provides a mechanism to regulate the binding of FILIP1 to FLNc under mechanical stress conditions, contributing to the dynamic adaptation of muscle cells to mechanical strain.