Fischer Group (Macromolecular Machines)
Welcome to the Fischer Group
The generation of mature messenger RNAs (mRNAs) and their translation into proteins depends on the elaborate interplay of a large number of trans-acting factors in eukaryotes. These factors are often organized in functional units (termed macromolecular machines), which catalyze the sequential steps in mRNA metabolism and timely coordinate their progression. Using a combination of biochemistry and structural biology (single particle cryo-electron microscopy and X-ray crystallography) our group studies the functional dynamics of key complexes acting on mRNA, and how their malfunction causes human diseases. The following topics are being investigated in our group:
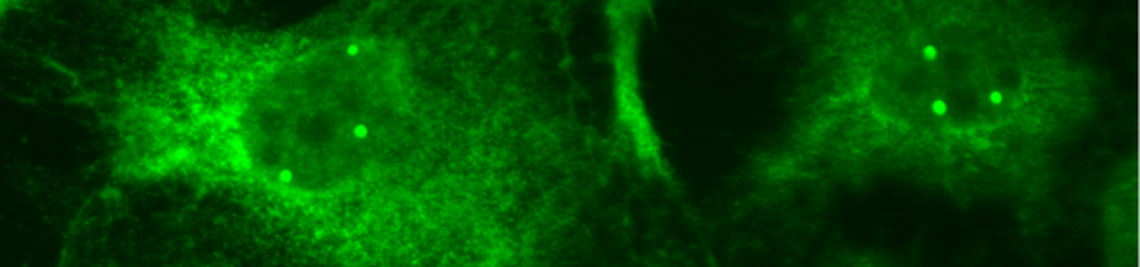
In this research focus we aim to understand how RNA-protein complexes (RNPs) are assembled within the context of a living cell. To address this question, we use the small nuclear ribonucleoprotein (snRNP) particles of the spliceosome as a model system. We have identified a complex network of assembly factors (“assembly chaperones”) that guide and facilitate RNP formation. Our current focus is the functional and structural investigation of the assembly machinery.
The conversion of genetic information into proteins is often tightly regulated by factors that interact with the mRNA. A prime example for such a regulatory event is the generation of proteins associated with the translational apparatus (e. g. ribosomal proteins or elongation factors), which is regulated by mitogenic and nutritional signals. The identification of factors and mechanisms governing the translation of this class of mRNAs is the major task of this project.
Defects in RNA-metabolic processes are the cause of many human diseases such as spinal muscular atrophy (SMA), spinal muscular atrophy with respiratory distress (DSMA1) or retinitis pigmentosa (RP). These diseases are the result of mutations in ubiquitously expressed genes with roles in translation (DSMA1) or pre-mRNA splicing (SMA, RP). In this research focus we aim to understand how mutations in these general mRNA-metabolic factors result in the tissue specific defect observed in the respective diseases. These studies comprise the generation of animal disease models and the genome-wide analysis of defects in mRNA metabolism.
A detailed insight into the structure of biological macromolecules and complexes is often the key for understanding their function. In this research focus we use X-ray crystallography to determine the atomic structure of protein and protein-RNA complexes involved in RNA metabolism (see also the projects of the research group "Macromolecular Crystallography" (C. Grimm/U. Fischer)).
Key publications:
The Ribosome Cooperates with the Assembly Chaperone pICln to Initiate Formation of snRNPs.
Paknia E, Chari A, Stark H, Fischer U.
Cell Reports 16(12):3103-3112 (2016)
Reconstitution of the human U snRNP assembly machinery reveals stepwise Sm protein organization
Neuenkrichen, N., Englbrecht, C., Ohmer, J., Ziegenhals, T., Chari, A., and Fischer, U.
EMBO Journal 34(14):1925-41 (2015)
Structural Basis of Assembly Chaperone- Mediated snRNP Formation
Grimm C, Chari A, Pelz J, Kuper J, Kisker C, Diederichs K, Stark H, Schindelin H, Fischer U
Molecular Cell 49(4):692-703 (2013)
Intronic miR-26b controls neuronal differentiation by repressing its host transcript, ctdsp2
Dill H, Linder B, Fehr A, Fischer U
Genes & Development 26(1):25-30 (2012)
An assembly chaperone collaborates with the SMN complex to generate spliceosomal SnRNPs
Chari A, Golas M, Neuenkirchen N, Klingenhäger M, Sander B, Englbrecht C, Sickmann A, Stark H, Fischer U
Cell 135:497-509 (2008)
Reduced RNP assembly causes motor axon degeneration in an animal model for spinal muscular atrophy
Winkler C, Eggert C, Gradl D, Meister G, Giegerich M, Wedlich D, Fischer U
Genes & Development 19:2320-2330 (2005)
A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs
Meister G, Bühler D, Pillai R, Lottspeich F, Fischer U
Nature Cell Biology 3:945-949 (2001)
The SMN-SIP1 complex has an essential role in spliceosomal U snRNP biogenesis
Fischer U, Lui Q, Dreyfuss G
Cell 90:1023-1029 (1997)
The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs
Fischer U, Huber J, Boelens W, Mattaj IW, Lührmann R
Cell 82, 475-483(1995)
Fischer, U. and Lührmann, R.
An essential signalling role for the m3G cap in the transport of U1 snRNP to the nucleus.
Science 249: 786-790 (1990)
For a complete list please click here.
Key reviews:
Chari, A. und Fischer, U.
Cellular strategies for the assembly of molecular machines.
Trends in Biochemical Sciences (TIBS) 35(12):676-83 (2010)
Gehring N, Wahle E, Fischer U.
Decipering the mRNP code: RNA-bound determinants of post-transcriptional gene regulation.
Trends in Biochemical Sciences (TIBS), DOI:10.1016/tibs.2017.02.004 (2017)