More: Poxviral Molecular Transcription Machines

Poxviral transcription initiation
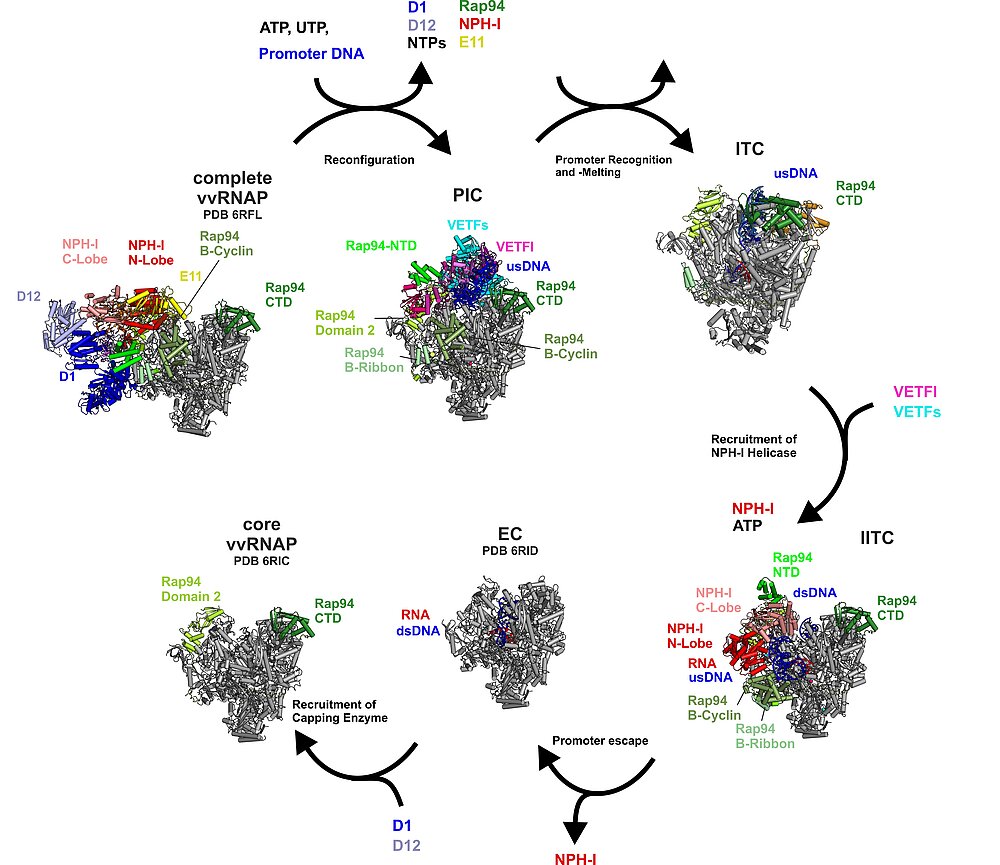
The Transcription Initiation Cycle
Complete vvRNAP, a putative packaging unit, reconfigures to the PIC (step 1). In the PIC, vRNAP-bound VETF positions and melts the promoter DNA. Upon handover of the melted promoter to the core polymerase, VETF leaves the complex, giving rise to the lPIC (step 2). Through the interaction with the phosphorylated C-tail domain of Rpo30, the B-homology domain of Rap94 is kept in an initiation-ready conformation. After single strand capture (step 3), the B-reader scans the template strand for the transcription start site, the B-homology domain becomes mobile and RNA synthesis commences (step 4). Promoter escape is accompanied by recruitment of NPH-I, a large-scale remodelling of Rap94, and major changes to the path of the upstream DNA (step 5). In the lITC complex NPH-I acts as a strand-separating helicase, widens the transcription bubble, defines its upstream fork point, and shapes the path of the single-stranded template- and non-template DNA. Transition to a processive elongation complex (step 6) triggers contraction of the transcription bubble, mobilization of the upstream DNA duplex and loss of NPH-I.
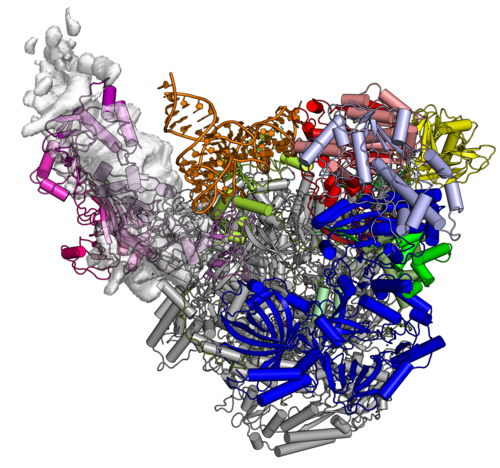
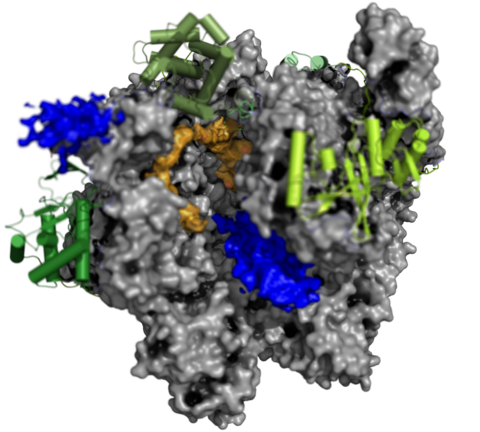
The Complete Vaccinia RNA Polymerase Complex
The vRNAP core enzyme resembles eukaryotic RNA polymerase II (Pol II), but also reveals many virus-specific features, including the transcription factor Rap94. The complete complexe comprises the core enzyme and additionally contains several virus-exclusive factors: the early viral transcription factor VETF, the mRNA processing factors VTF/CE, the helicase NPH-I, the viral core protein E11 and, surprisingly, a host tRNA molecule . This complex can carry out the entire early transcription cycle.
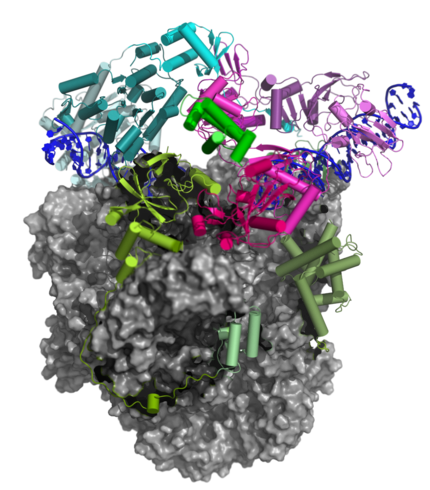
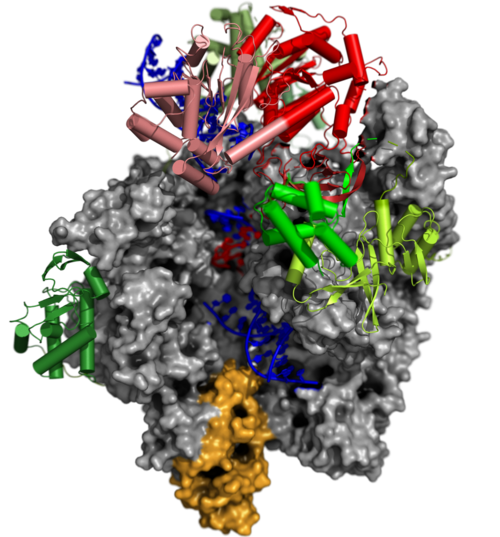
Transition of the Complete vRNAP to the Pre-Initiation Complex (PIC)
The Pre-Initiation Complex (PIC)
The early transcription factor (VETF) adopts an arc-like shape, spans the polymerase cleft and anchors upstream and downstream promoter elements. Four domains of the large subunit (VETFI) cooperate in upstream promoter recognition, enforcement of transcription directionality, and PIC stabilization. A fifth domain adopts a TATA binding protein-like fold that inserts asymmetrically into the DNA major groove, and triggers bending and melting of promoter DNA. the small subunit (VETFs), which displays a helicase fold that contacts the downstream promoter, induces a sharp bend in the DNA helix and fosters the initial melting event around the transcription start site.The structure comprising the first bilobal TBP-like protein solved thus far and visualizes the promoter in the initially melted state. That means thatm VETF has just melted the promoter, and it has entered the cleft where it is stabilized by the clamp head and the lobe domain of the polymerase core.
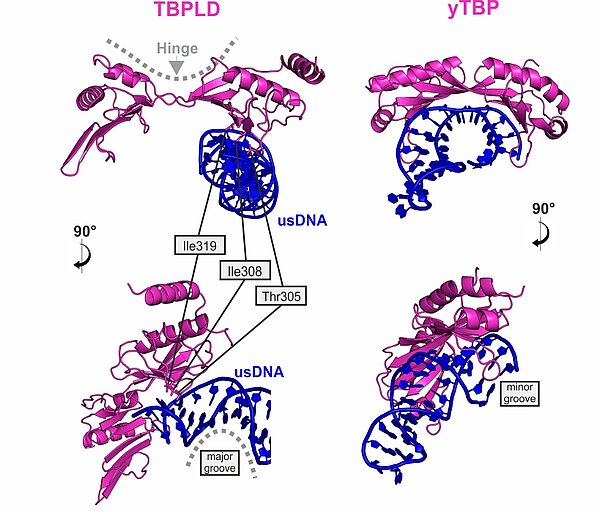
VETFl Contains a TBP-like Domain that Displays a Non-Canonical Binding Mode
The VETFl sequence does not display any detectable homology. Nevertheless it contains a domain with a bilobal TBP fold. When compared to the TBP/TATA crystal structure, it is obvios that its binding mode is fundamentally different from genuine TBP.
The Late PIC
The late PIC iss unique in that a phosphorylated tail at a core subunit binds into the active cleft. Here, it locks the B reader element of transcription factor Rap 94 in an initiation-ready conformation by imitating the template strand. During single-strand capture, the phosphopeptide is eventually displaced.
The Late Initially Transcribing Complex - A High Power Scrunching Machine
For promoter escape, the initially transcribing complex undergoes a dramatic rearrangement and re-acquires the helicase transcription factor NPH-I. The main function of NPH-I in this complex is to act as a motor that 'scrunches' upstream DNA into the cleft to produce an energy-loaded intermediate.