Algal Blue Light Switch Control of Electrical Excitation in Plants
08/10/2020What is the role and molecular basis of electrical signaling in higher plants? This can now be investigated non-invasively for the first time. The new method has been published in the journal PNAS.
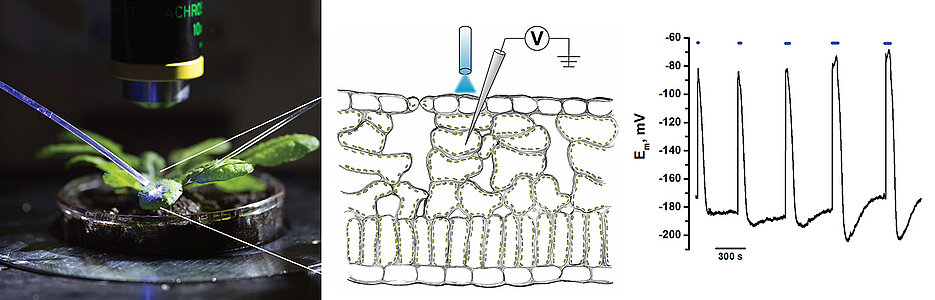
Optogenetics denotes the manipulation of cellular processes by light-based biological techniques. An international research team led by the Würzburg plant scientists Rainer Hedrich, Georg Nagel and Dirk Becker has succeeded in applying this method to higher plants: Light impulses can now be used to trigger electrical excitation in plants.
"With this tool, for the first time we are able to non-invasively investigate electrically based cellular communication pathways in plants at the molecular level and ask how plants use these electrical signals to respond to extreme temperature fluctuations, herbivores or other stress factors," says Dirk Becker.
When plants are stressed, they emit long distance travelling electrical signals known as membrane potential waves. Thereby, plants are capable to transmit information quickly and precisely over long distances, even though they have neither brain nor nerve cells. The molecular mechanisms involved, however, are largely unknown. New insights into these complex processes are provided by the research team in the renowned journal PNAS (Proceedings of the National Academy of Sciences USA).
Algae provide tools for membrane biology
How can we simulate an electrical signal in plants that is normally triggered by stress or injury without causing unwanted side reactions?
The team tackled this challenge with the help of optogenetics. The method has been available since 2002 and the co-authors of the current PNAS publication, Georg Nagel and Ernst Bamberg, together with other researchers, have received several awards for their development.
Optogenetics allows to control the electrical activity of nerve cells with light pulses, provided that nerve cell membranes were previously equipped with light-sensitive ion channels from algae, known as channelrhodopsins.
Stress leads to depolarisation and acidification
Higher plants have lost the light-sensitive ion channels of algae during evolution, explains Dirk Becker. The researchers have now succeeded in returning the channelrhodopsin genes back to the genome of the model plant Arabidopsis thaliana, whose leaf cells can be specifically excited with light and the membrane electric response can be analysed.
If plants are stressed, irritated cells depolarise and the cell environment becomes more acidic. This was known. But how can the two processes be simulated in the experiment? The Würzburg researchers use a channelrhodopsin variant that is switched on by blue light and then conducts protons into the cell.
Normally, the cell wall of a plant cell is at least one pH unit more acidic than the cell interior, says Rainer Hedrich. If the proton channel opens, protons and hence positive electric charges inevitably flow across the cell membrane. This depolarises the membrane and acidifies the cell interior.
Depolarisation can be controlled by blue light
In order to trigger this effect experimentally, a blue laser is directed at the leaf area to be investigated and the membrane potential of the stimulated cells is recorded, explains Dirk Becker: "We used the illumination intensity, duration and frequency of the blue light pulses to control the shape of the membrane depolarisation and analysed the repolarisation response of the plant cell in detail.
It was shown that in leaf cells the repolarisation is mainly caused by ATP-driven plasma membrane potential-sensitive proton pumps. When the cell membrane depolarises, this proton pump enters a state of increased activity. In doing so, it transports more positively charged protons out of the cell, which repolarizes the cell membrane.
This mechanism differs fundamentally from that in animal nerve cells, where voltage-dependent potassium channels govern this process. The Würzburg plant researchers were able to show that plants manage this process using a proton pump and not a potassium channel: An Arabidopsis mutant without potassium channel behaved like a normal plant when exposed to blue light.
Channelrhodopsins for all cases
"We are currently testing other optogenetic tools of this kind," says Rainer Hedrich. The aim is not only to elucidate cellular communication through electrical signals. It is also important to understand the importance of calcium waves and pH signals occurring simultaneously in plants.
In order to clarify what characterises plant cells in general and which cell-specific characteristics may have developed, the researchers plan to introduce channelrhodopsins into cells exhibiting a broad range of different functions. The researchers also plan to use channelrhodopsin variants with specific ion selectivity to shed light on the intricate communication pathways of plants.
Publication
"Channelrhodopsin-mediated optogenetics highlights a central role of depolarization-dependent plant proton pumps", PNAS, August 10, 2020, https://www.pnas.org/cgi/doi/10.1073/pnas.2005626117
Contact persons
Prof. Dr. Dirk Becker, Chair of Botany I (Plant Physiology and Biophysics), University of Würzburg, T +49 931 31-86108, dbecker@botanik.uni-wuerzburg.de
Prof. Dr. Rainer Hedrich, Chair of Botany I (Plant Physiology and Biophysics), University of Würzburg, T +49 931 31-86100, hedrich@botanik.uni-wuerzburg.de