Matchmaking with consequences
10/16/2017Myc proteins play an important role when cells become cancerous. Researchers from the University of Würzburg have studied just how they do this. They might thus open up ways to develop new therapies.
Most human tumours have one thing in common: They harbour drastically increased amounts of the so-called Myc proteins. Animal experiments show that such high Myc concentrations contribute to causing cancer. But Myc proteins not only have harmful properties, they are also crucial in healthy cells: Acting as "transcription factors", they control the activity of a limited number of genes. A lot of these genes activated by Myc are essential for the growth and proliferation of normal cells so that Myc is indispensable for normal human development.
How exactly Myc proteins work inside tumour cells has been unknown so far. Scientists from the University of Würzburg have now uncovered key details of these processes. The study is led by Dr Peter Gallant, team leader at the Department of Biochemistry and Molecular Biology. The researchers present the results of their work in the current issue of the journal PNAS - Proceedings of the National Academy of Sciences.
Excessive Myc levels cause malignancy
"Myc proteins bind to their target genes through well-defined molecular interactions which typically contain an exactly defined sequence of nucleotides," Peter Gallant explains the basics of his study. But when Myc proteins occur in much higher concentrations, they will bind to virtually all active genes and thereby further amplify the activity of these genes, which then contributes to the affected cell becoming cancerous.
"Such behaviour is highly unusual for transcription factors and was impossible to explain in molecular terms so far," the biochemist details. According to Gallant, scientists have previously been baffled by the question of how a transcription factor, which usually only recognises short nucleotide sequences, can bind to all genes – even to such genes that do not contain this nucleotide sequence in the first place.
An enzyme acts as facilitator
The researchers found the explanation when they studied other proteins that also bind to all genes –the enzymes that read all genes and transcribe them to RNA: RNA polymerases and the associated auxiliary proteins that are important for polymerase activity. This includes the "polymerase associated factor 1" complex, short PAF1 complex, which consists of five proteins. It provided Gallant and his team answers to Myc's unusual behaviour.
The researchers used fruit flies (Drosophila melanogaster) as the model system for their studies. "Myc proteins in these insects work very similarly to mammals, but the corresponding experiments are easier and more efficient to conduct here," Gallant explains. A genetic screen showed that the PAF1 complex is important for the Myc activity – in particular for Myc's ability to activate specific target genes and to stimulate cell growth.
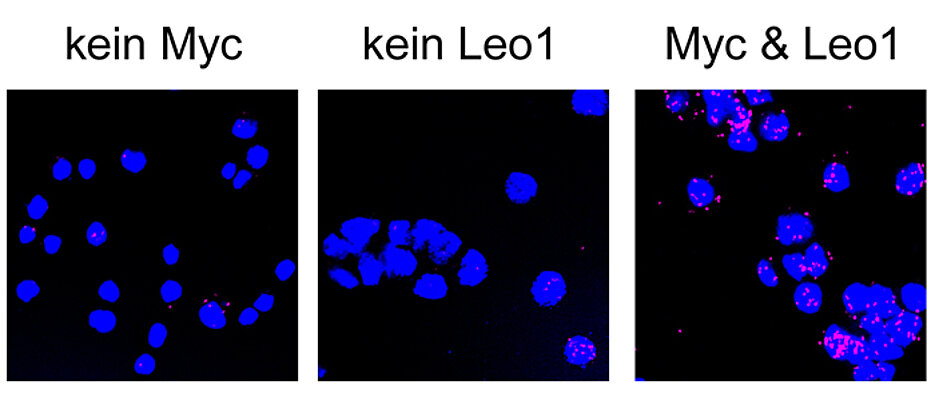
Advanced biochemical and molecular biological analyses, including genome-wide sequence analyses – the so-called "next-generation sequencing" – then shed light on the functioning of the PAF1 complex: Being a "polymerase-associated factor, this complex is located at virtually all active genes. At the same time, it is capable of binding to the Myc protein. "This recruits Myc proteins to active genes and can further boost their activity," Gallant explains. This stimulating effect seems to be important especially at elevated Myc levels.
Additional factors at play
In their experiments, the researchers demonstrated that while the destruction of the PAF1 complex significantly weakens the bond of the Myc proteins to their target genes, it does not eliminate it completely. "This points to the fact that there are other factors that contribute to the recruiting of Myc similarly to the PAF1 complex," Gallant explains and he says that another protein was recently identified which has similar basic functions when reading genes as the PAF1 complex and which simultaneously also binds to Myc and recruits this protein to numerous genes.
Therefore, the scientists assume that more such general factors exist that contribute to Myc's binding to all genes, and which are important particularly for abnormally elevated Myc levels.
New approach for drug development
The Würzburg scientists believe that their findings are not only of biochemical but also of medical relevance as Myc proteins play a key role in cancer development. "So far it has been impossible to develop drugs that specifically inhibit the activity of Myc proteins," Gallant explains. He is hopeful that the newly discovered interactions present potential new targets to develop drugs that selectively block Myc.
PAF1 complex component Leo1 helps recruit Drosophila Myc to promoters. Jennifer Gerlach, Michael Furrer, Maria Gallant, Dirk Birkel, Apoorva Baluapuri, Elmar Wolf, Peter Gallant. PNAS Early Edition, published online October 16. www.pnas.org/cgi/doi/10.1073/pnas.1705816114
Contact
Dr. Peter Gallant, Department of Biochemistry and Molecular Biology
Phone: +49 931 31-88814, peter.gallant@biozentrum.uni-wuerzburg.de