New gene mutation associated with Fanconi anemia
07/14/2017Fanconi anemia is a rare genetic disease characterized by high cancer risk. Researchers of the University of Würzburg now have revealed a new Fanconi anemia gene that is involved in complex DNA repair processes and may also play a relevant role in cancer prevention.
Fanconi anemia is a rare genetic disease characterized by bone marrow failure heralded by low platelet counts and unusually large red blood cells. Mutations in over 20 genes have been identified as causative for Fanconi anemia, which encode proteins commonly involved in DNA repair mechanisms.
The failure to repair DNA is considered the source of increased cancer risk in individuals with Fanconi anemia. Ongoing efforts to identify additional genes and pathways linked to this disease may concurrently reveal potential susceptibility genes for hereditary cancers.
This week in the Journal of Clinical Investigation (JCI), a team led by Detlev Schindler at the Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, reports classical Fanconi anemia symptoms in a 12-year-old individual without mutations in any of the known Fanconi anemia genes.
Mutations detected
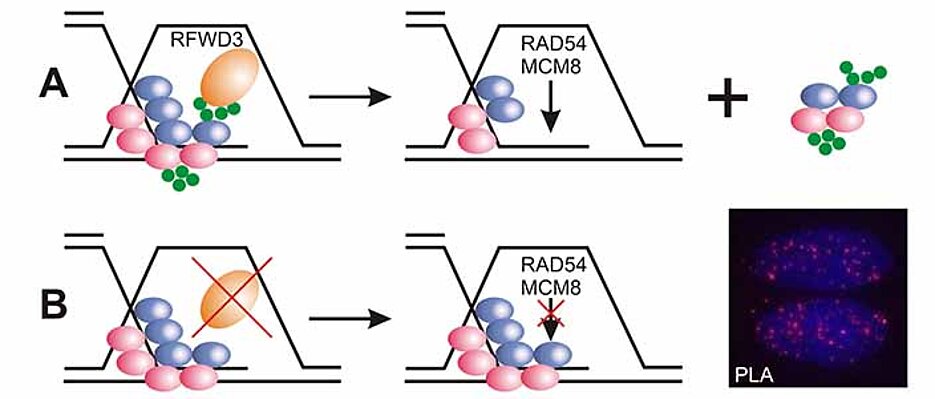
Sequencing of this individual’s genome detected mutations in both alleles of the gene RFWD3, which encodes an enzyme that helps target other proteins on single-stranded DNA for degradation. This process is impaired in patient’s cells which rendered them more sensitive to chromosome breakage and DNA damage, compared to cells from healthy individuals.
Other cells either lacking RFWD3 or genetically engineered with the patient’s missense mutation showed similar DNA repair defects, which were rescued by expression of wild-type RFWD3. Moreover, RFWD3-deficient mice exhibited a phenotype that resembles other mouse models of Fanconi anemia. Together, these findings support the identification of RFWD3 as a Fanconi anemia gene.
„Biallelic mutations in the ubiquitin ligase RFWD3 cause Fanconi anemia“, Kerstin Knies, Shojiro Inano, María J. Ramírez, Masamichi Ishiai, Jordi Surrallés, Minoru Takata, and Detlev Schindler. Journal of Clinical Investigation, 10. July 2017, DOI: 10.1172/JCI92069,
http://www.jci.org/articles/view/92069?key=85a36008596dbac1b621http://www.jci.org/articles/view/92069
Schindler and collaborators further describe the mechanisms by which RFWD3 mediates DNA repair in two accompanying studies recently published in Molecular Cell. Future explorations of this enzyme may reveal its importance as a therapeutic target in certain subtypes of Fanconi anemia or cancer.
The publications in „Molecular Cell“
https://doi.org/10.1016/j.molcel.2017.04.022
http://dx.doi.org/10.1016/j.molcel.2017.04.021
Contact
Prof. Dr. Detlev Schindler, Institute of Human Genetics, University of Würzburg, Germany, T +49 931 31-88075, schindler@biozentrum.uni-wuerzburg.de