Research Projects
Control of Cdc48 function by cofactors
The essential and highly conserved ATPase Cdc48 (also known as p97 and VCP in mammals) is a motor and regulator of many ubiquitin-controlled cellular processes. It is involved in the proteasomal degradation of protein quality control targets, cell cycle regulators, transcription factors and DNA repair proteins, but also in non-proteasomal proteolysis through macroautophagy and the endolysosomal pathway. Moreover, Cdc48 mediates the non-proteolytic mobilization of ubiquitylated proteins, e.g. transcriptional regulators and SNARE proteins.
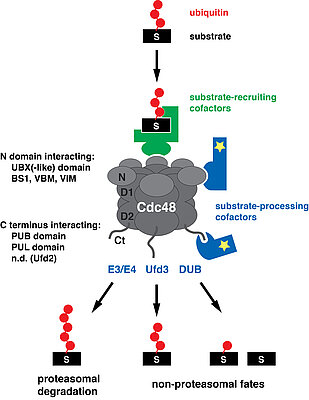
Cdc48 is a member of the AAA (ATPases Associated with diverse cellular Activities) protein family and forms homohexameric, ring-structured complexes. It uses energy derived from ATP hydrolysis to extract or “segregate” ubiquitylated target proteins from stable protein assemblies, membranes and chromatin. Because Cdc48 is involved in such diverse cellular processes, its segregase activity must be tightly controlled. This is achieved by a large number of regulatory cofactors, which control substrate specificity and fate as well as subcellular localization and oligomeric state of Cdc48.
The main research focus of our group is the identification and functional characterization of Cdc48 cofactors in order to obtain novel insights into physiological functions of Cdc48 and their pathogenic impairment in human disease.
Identification of Cdc48 cofactors
The majority of cofactors interacts with Cdc48 through a small number of conserved binding modules. We identified three major binding modules, i.e. the UBX domain (Buchberger et al., 2001; Schuberth et al., 2004), the PUB domain (Allen et al., 2006), and the short, linear VIM binding motif (Stapf et al., 2011), which enabled the identification of a significant fraction of all known cofactors. In particular, UBX domain containing proteins represent by far the largest group of cofactors. In collaboration with bioinformaticians and structural biologists, we continue to search for novel Cdc48 binding modules and cofactors.
Functional characterization of Cdc48 cofactors
We study the cellular function of Cdc48 cofactors in the model organism Saccharomyces cerevisiae (baker´s yeast). In the past, we uncovered important roles for the UBX proteins Ubx2 and Shp1 in ER-associated protein degradation (ERAD) (Schuberth & Buchberger, 2005) and in the regulation of protein phosphatase 1 (Böhm & Buchberger, 2013), respectively. We also showed that cellular functions of the substrate-processing cofactors Ufd2 and Ufd3 in the ubiquitin proteasome system require their association with Cdc48 (Böhm et al., 2011). Taking advantage of the well established genetic, biochemical and cell biological approaches to study baker´s yeast, we are currently exploring the functions of further cofactors. Recently, we also started to characterize human p97 cofactors with presumed metazoan-specific functions, for example in NF-κB signaling.
Elucidation of pathogenic mechanisms in p97-associated proteinopathies
Missense mutations in the human VCP gene encoding p97 are causative of two fatal protein aggregation diseases ("proteinopathies"), Inclusion Body Myopathy with Paget´s disease of the bone and Fronto-temporal Dementia (IBMPFD) and familial Amyotrophic Lateral Sclerosis (fALS). On the cellular level, both diseases are characterized by cytoplasmic protein inclusions (aggregates) positive for ubiquitin and the Tar DNA-binding Protein 43 (TDP-43). Analysis of IBMPFD patient-derived cell-lines and IBMPFD mouse models revealed that disease-causing p97 mutant proteins are impaired in autophagy and endolysosomal protein degradation. On the molecular level, we were able to demonstrate that these p97 variants exhibit altered interactions with several cofactors (Fernandez-Saiz & Buchberger, 2010). Our aim is to elucidate the consequences of these molecular defects for the p97 segregase activity and the turnover of critical cellular substrates.
Cited Publications
Allen M, Buchberger A and Bycroft M (2006). The PUB domain functions as a p97 binding module in human peptide N-glycanase. J Biol Chem 281:25502-25508.
Böhm S, Lamberti G, Fernández-Sáiz V, Stapf C and Buchberger A (2011). Cellular functions of Ufd2 and Ufd3 in proteasomal protein degradation depend on Cdc48 binding. Mol Cell Biol 31:1528-1539.
Böhm S and Buchberger A (2013). The budding yeast Cdc48-Shp1 complex promotes cell cycle progression by positive regulation of Protein Phosphatase 1 (Glc7). PLoS One 8:e56486.
Buchberger A, Howard MJ, Proctor M and Bycroft M (2001). The UBX domain: a widespread ubiquitin-like module. J Mol Biol 307:17-24.
Fernández-Sáiz V and Buchberger A (2010). Imbalances in p97 co-factor interactions in human proteinopathy. EMBO Rep 11:479-485.
Schuberth C, Richly H, Rumpf S and Buchberger A (2004). Shp1 and Ubx2 are adaptors of Cdc48 involved in ubiquitin-dependent protein degradation. EMBO Rep 5:818-824.
Schuberth C and Buchberger A (2005). Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated degradation. Nat Cell Biol 7:999-1006.
Stapf C, Cartwright E, Bycroft M, Hofmann K and Buchberger A (2011). The general definition of the p97/valosin-containing protein (VCP)-interacting motif (VIM) delineates a new family of p97 cofactors. J Biol Chem 286:38670-38678.