Pediatric kidney tumor and Wilms tumor genetic
Kidney tumors are the second largest group of solid tumors in childhood. They are probably based on misdiagnosed embryonic kidney precursor cells. For Wilms tumors (also called nephroblastomas) we identified mutations in a number of genes as possible triggers. We try to understand the mechanism of action of these tumor genes through in vitro experiments and with the help of mouse models, in order to enable a more targeted diagnosis and to find new targets for improved therapies.
For this we establish cell cultures of these tumors and compare the function of normal and mutated gene copies. Using high-throughput methods, we search for differences in gene activity and in the biological behavior of the altered proteins.
WT1 (Wilms tumor suppressor gene 1) is the first tumor suppressor gene we have discovered to play a role in the development of Wilms' tumors. The WT1 protein is a transcription factor that regulates important processes, among others, in the embryonic kidney development controls. In about 15% of all Wilms tumors a mutation or a loss of WT1 can be detected.
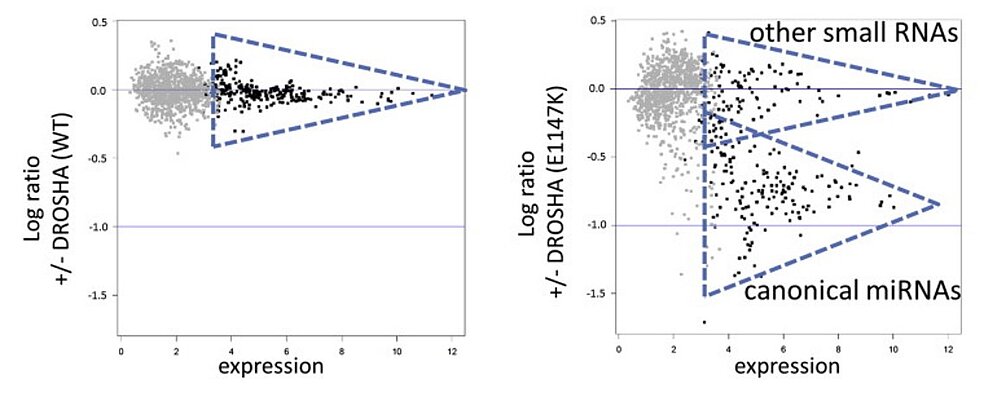
DROSHA and DGCR8 comprise the main components of the miRNA microprocessor complex which plays an important role in the biogenesis of miRNAs.
We and others identified recurrent somatic hotspot mutations in these genes with a high incidence in high-risk blastemal type Wilms tumors. Functional in vitro analysis showed that aberrations in these genes lead to a significant downregulation of canonical miRNAs illustrating their crucial role in miRNA processing. Since miRNA processing genes have been found to play an increasingly important role in pediatric tumors, we are interested in the further characterization of DROSHA and DGCR8 mutations as putative oncogenic drivers in Wilms tumorigenesis.
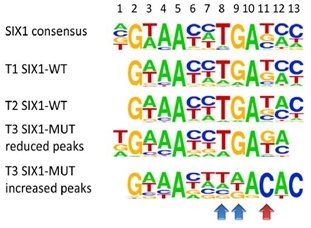
SIX1 (SIX homeobox 1) and SIX2 belong to the SIX protein family and are key transcription factors for early kidney development. They are necessary to maintain the mesenchymal progenitor cells, which give rise to functional units of the kidney. Loss of any of these genes leads to kidney agenesis or reduced size. On the other hand, Six1 and Six2 are part of a gene set that allows reprogramming of adult kidney tubule cells into nephron progenitors.
Recently, we found recurrent mutations of the DNA binding domain of SIX1 and SIX2 in high-risk blastemal WT, that we are now analyzing in corresponding mouse models.
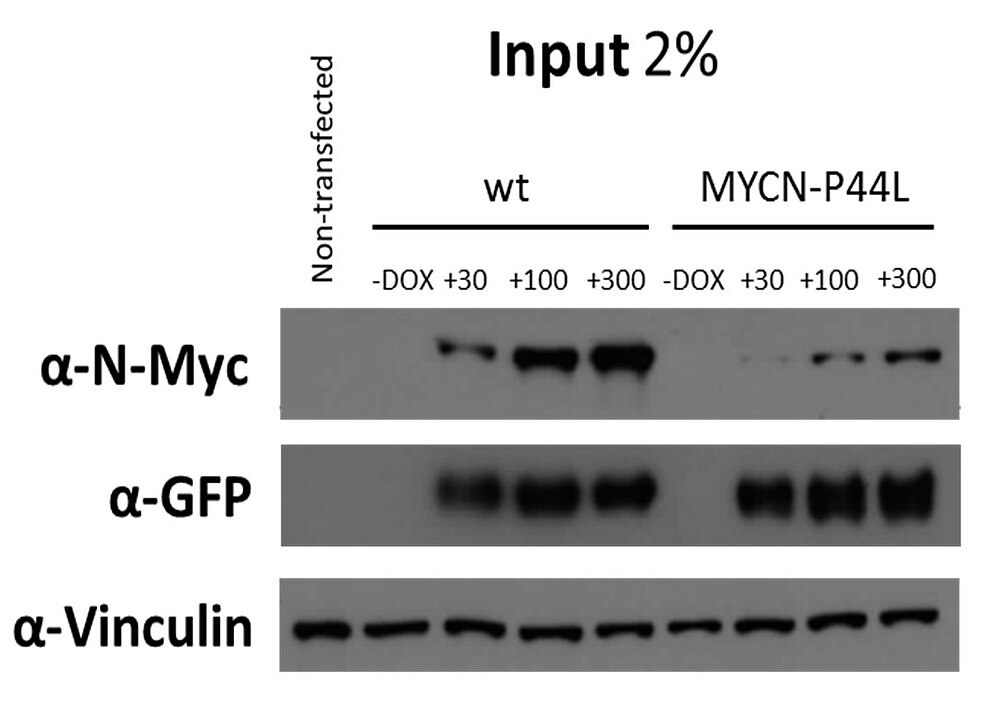
N-myc is a proto-oncogene and a member of the Myc family of bHLH transcription factors, and plays a major role in many vital cellular processes.
Its overexpression is associated with a variety of tumors – including Wilms tumor, where genomic MYCN gain and MYCN overexpression is associated with poor prognosis. In addition, MYCN P44L mutation was found in some cases of WT. To further characterize these events in WT, we are characterizing MYCN (and its interaction partner MAX) mutations in-vitro, and establishing a WT in-vivo model for functional studies of MYCN overexpression.
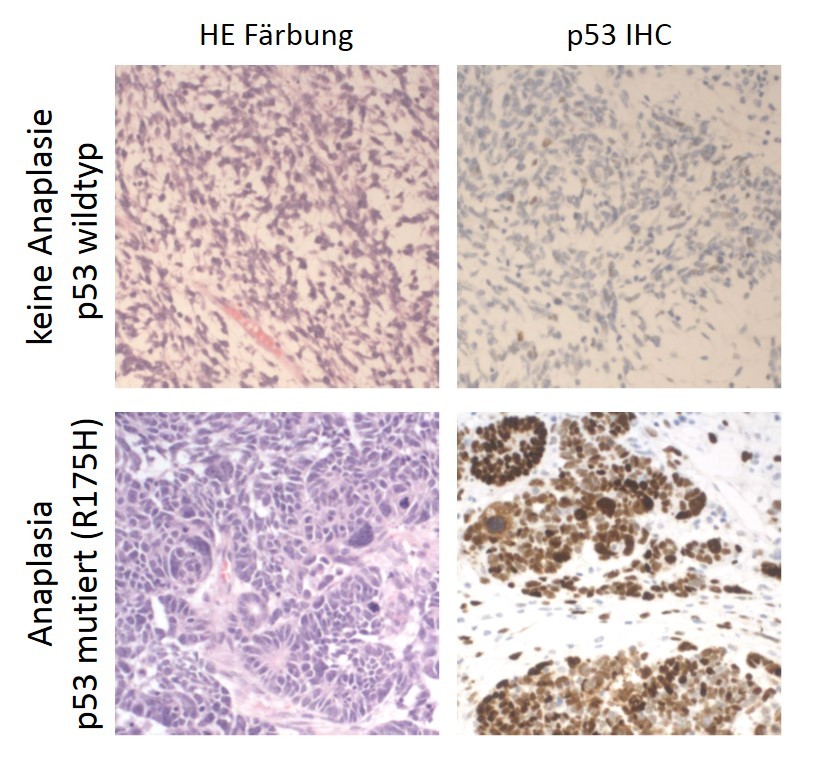
Also referred to as "guardian of the genome", p53 plays an important role in genome stability by preventing cells with DNA damage from cell division. We find p53 mutations mainly in anaplastic Wilms tumors that belong to the high-risk group of this tumor. We are currently investigating whether p53 mutations occur outside of this subgroup and provide diagnostic value pointing towards aggressive disease.


![[Translate to Englisch:] header Mikroskopierraum](/fileadmin/_processed_/4/e/csm_header_mikroskop_7b0513dec1.jpg)