Projects
Regulation of stomatal movement
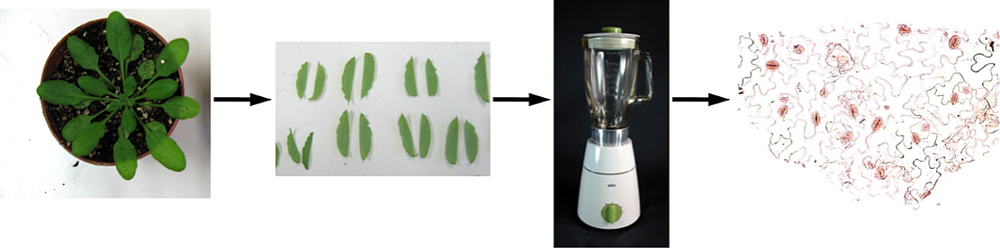
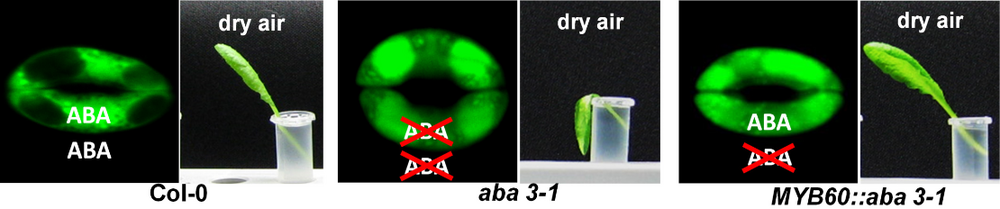
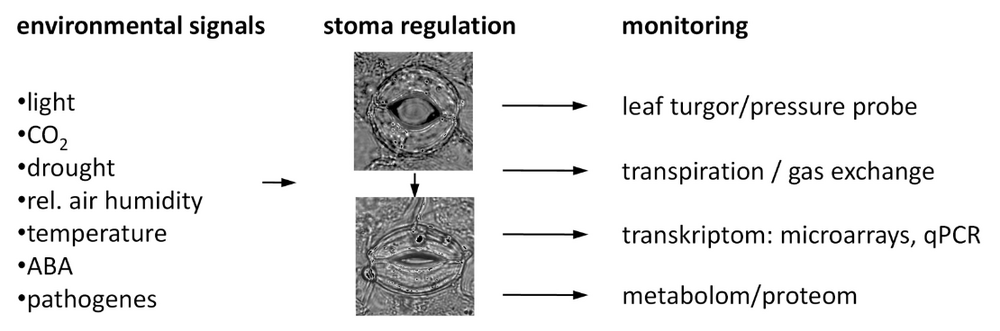
The world-wide climate change is expected to induce extreme fluctuations in temperature and humidity. One strategy of plants to survive the related physiological variations is to precisely adapt their water use efficiency. A key element in this process is the control of the gas exchange via the stomata, formed by two guard cells. Thereby the open state of the stomatal pore is highly regulated by turgor driven volume changes of the guard cells. The fine tuning of these biological valves is triggered by several internal and external factors, which are initially detected by a multi-sensory network and then translated into the appropriate movement of the stomatal pore. Up to now only a few of the involved signal transduction pathways have been characterized in detail. However, overlaps and interferences between the individual pathways, when e.g. different signals act simultaneously or in short intervals, have so far rarely been investigated. To address this question we focused on the transcriptional analysis of stomatal guard cells. First we optimized our procedure to mechanically sample enriched intact guard cells that avoids the possible side-effects (mainly osmotic stress, loss of tugor, and/or exposure to potential fungal elicitors) of the commonly used protoplasting method. Therefore total leaves without petiole and major veins are mechanically disrupted by successive blender cycles. Guard cells represent the only living cells of the resulting epidermal fragments (Figure 1). These samples are then used for the subsequent analyses of expression patterns (by micro arrays or qPCR), metabolites or proteins. To identify genes enriched in guard cells (compared to total leaves) and genes responding to several stimuli that lead to stomatal closure, we conducted a series of microarray experiments (Figure 2). Thereby we could show that e.g. guard cells can directly detect drought via changes in the relative air humidity and that they are able to respond by autonomous induction of the stomatal closure (Figure 3). Nevertheless, the nature of the humidity sensing mechanism, its conversion into a chemical signal and finally into the mechanical stomatal movement is so far unknown and focus of our current research interest. We have started a bioinformatics approach to identify the interacting nodes between single signalling pathways. By use of marker genes and physiological treatments of the respective Arabidopsis mutants we will unravel the whole signal transduction network of stomatal closure step by step.
Our main methods:
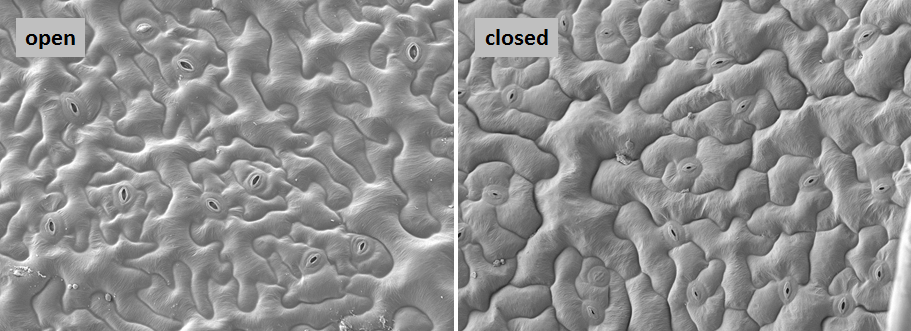
We use physiological methods to determin the open state of the stomata following application of several closing signals, and thus the appropriate time point for sampling (Figure 4 und 5).
Physiology
- measurement of stomatal movement via transpiration (gas exchange, IRGA)
- measurement of stomatal movement via leaf turgor (non-invasive pressure probe)
- preparation of intact guard cells
- ELISA-monitoring of the stress hormone ABA
Molecular biology/Expression analysis:
- standard methods of molecular biology
- preparation of low amount RNA
- quantitative real-time-PCR (qPCR)
- microarray analyses