Improved optogenetic tools
Improved optogenetic tools: Characterization of new rhodopsins and engineering of novel features into existing photoreceptors (funded by TR166 until June 2019)
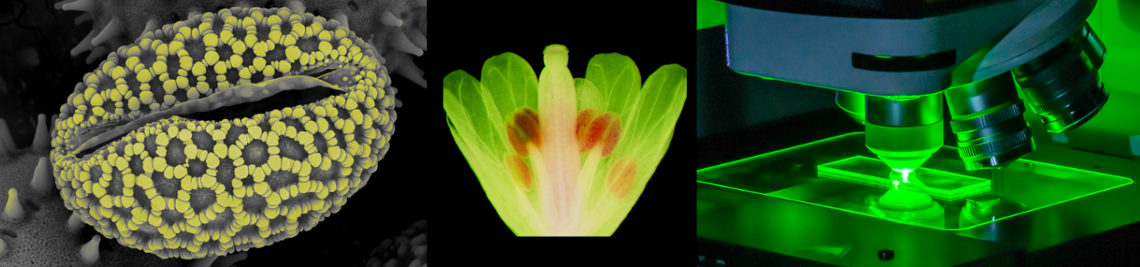
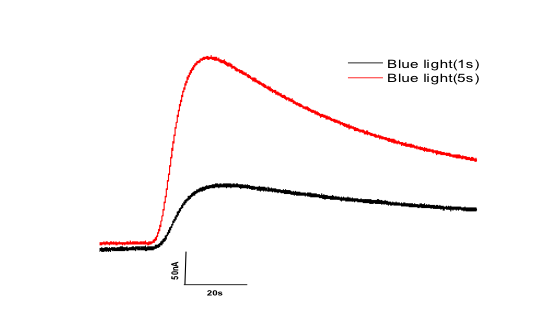
The breakthrough for “optogenetic actuators” came with the discovery of channelrhodopsin-2, a directly light-gated cation channel, able to depolarize cells and to light-modulate behaviour of transgene animals. The success of channelrhodopsin-2 encouraged us to introduce halorhodopsin as a hyperpolarizing and “neuron-inhibiting” optogenetic protein. Halorhodopsin, as well as Channelrhodopsin, however, have certain limitations due to their relatively small charge transfer per protein molecule and their requirement for high light intensity and high protein expression. In 2014, we introduced a Channelrhodopsin-2 mutant with improved expression and dramatically increased photocurrents, even at low light intensity (ChR2/XXL, Dawydow et al., 2014, PNAS). In this project we develop improved optogenetic actuators with larger charge transfer per molecule and lower light intensity requirement, based on engineering of established optogenetic tools and characterization of new photoreceptors. We are coupling cyclic nucleotide-gated (CNG) cation or CNG potassium (CNGK) channels to light-activated adenylyl or guanylyl cyclases. Compared to channelrhodopsin, these channels show higher single channel conductance. Once made light-sensitive, then even sparse expression (compared to channelrhodopsin and halorhodopsin) will allow light-activated de- (CNG) or hyper-(CNGK) polarization. When expressed in Drosophila melanogaster, we could show the light-induced and fully reversible activation of such channels by short illumination of larvae (Beck et al., 2018).