Role of plasma membrane lipid nanodomains for plant cell signalling processes
Role of plasma membrane lipid nanodomains for plant cell signalling processes
Lipid nanodomains in the plasma membrane (also often referred to as “lipid rafts”) are spatially organised membrane patches structurally defined by an enrichment of certain lipids and proteins. In animal cells, such lipid microdomains have been extensively characterised as signaling platforms and in recent years evidence is emerging that membrane domains also play an important role in plant cell signaling.
We could already provide first evidence in plants that membrane microdomains might well serve as platforms for functional ion channel protein assembly and regulation during drought stress signalling mediated by the phytohormone abscisic acid (ABA): In Arabidopsis thaliana mesophyll cells, all ABA-signaling components regulating membrane-delimited steps downstream of the central protein phosphatase 2C ABI1 are at least transitionally associated with stable nanodomain platforms labeled by a lipid raft marker belonging to the family of remorin proteins (see picture). In the presence of ABA, the anion channel SLAH3 interacts with its phosphorylating kinase CPK21 within such platforms, which leads to activation of this channel. In the absence of the phytohormone, ABI1 blocks the formation of the CPK21/SLAH3-signaling complex at the plasma membrane and thereby prevents SLAH3 activation (see Demir et al. 2013 PNAS).
Another signaling protein identified as a putative nanodomain resident is the NADPH/respiratory burst Oxidase Protein D (AtrbohD). NADPH oxidases are an important source for the enzymatic production of ROS e.g. as second messenger after pathogen attack or contact with potent elicitors. Besides their function in pathogen defence, ROS have been shown to be also involved in ABA signalling, e.g. in Arabidopsis guard cells. We are investigating the role of the mesophyll-localized NADPH oxidase AtrbohD in mediating signal transduction events in this tissue and aim to reveal the functional significance of its association with membrane domains.
We are currently applying various methods to investigate the role of plant plasma membrane nanodomains:
- Molecular Biology: cloning, expression analysis (real-time qPCR)
- Biochemistry: Isolation of membrane subfractions, protein identification
- Proteomic analysis of nanodomain protein composition: Mass spectrometry, in close collaboration with Y. Reinders (University of Regensburg) and W.X. Schulze (University of Hohenheim)
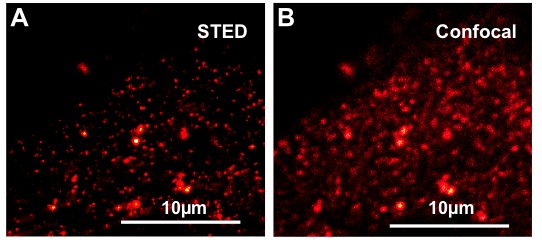
- Microscopic visualization of proteins and protein dynamics: Confocal microscopy, protein interaction studies (FRET, BIFC), Fluorescence Lifetime Imaging Microscopy (FLIM, FRET-FLIM), Stimulated Emission Depletion (STED) microscopy, Total Internal Reflection Fluorescence (TIRF) microscopy, in close collaboration with G.S. Harms (Wilkes University, Wilkes-Barre, USA)