Ion channels
Molecular mechanisms of ion transport in plant ion channels
Ion channels exhibit numerous essential functions in animal as well as plant cells. They are responsible for the maintenance of ion homeostasis, the regulation of cell volume and turgor, they control the cellular pH and they are part of inter- and intracellular transport of small molecules and ions with transport of the latter forming the basis of electric excitability of cells. Isolating the cells by a hydrophobic barrier (aka cell membrane) demands the existence of such pore-forming proteins to allow the exchange of charged molecules/ions. In order to control the ion flux as well as the nature of the passing ion certain architectural features must exist in the transmembrane protein such as gate, a selectivity filter and additional domains and modules that enable modulation of the properties of the former two features. particular technical difficulties in the recombinant production as well as the structure determination itself have -despite the progress made during the past 10 years- resulted in our still limited knowledge about molecular details of ion transport and its regulation.
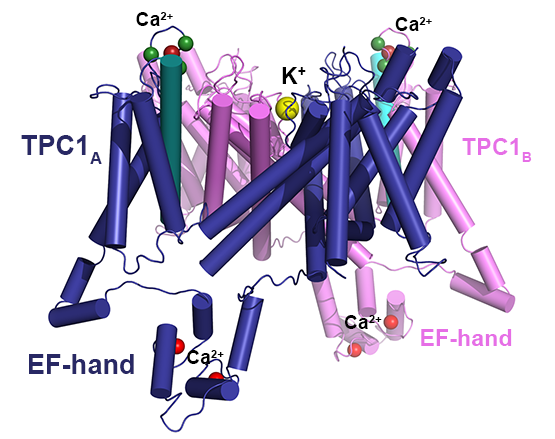
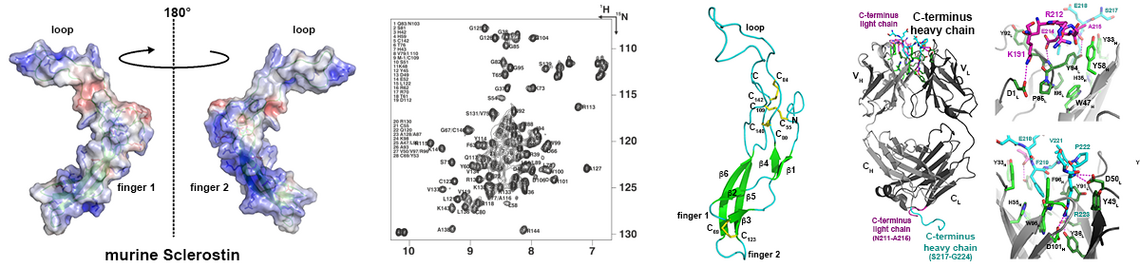
Our group is interested in the structure analysis of several members of the SLAC/SLAH anion channel family of plants (1). These ion channels are involved in the opening and closing of stomata and thus play an important role in the regulation of water loss in plants due to transpiration. Recombinant production of integral membrane proteins is still a rather difficult task requiring development and stringent optimization of expression and purification schemes. The recombinant membrane proteins shall then be crystallised and the crystals shall be analysed to yield X-ray diffraction data. Recent technical progress in cryo-electron microscopy rendered this methodology an alternative, as it may provide high-resolution structure data when certain conditions can be met. Since this method does not require protein crystals it can be even used when crystallization of our protein failed despite optimization trials.
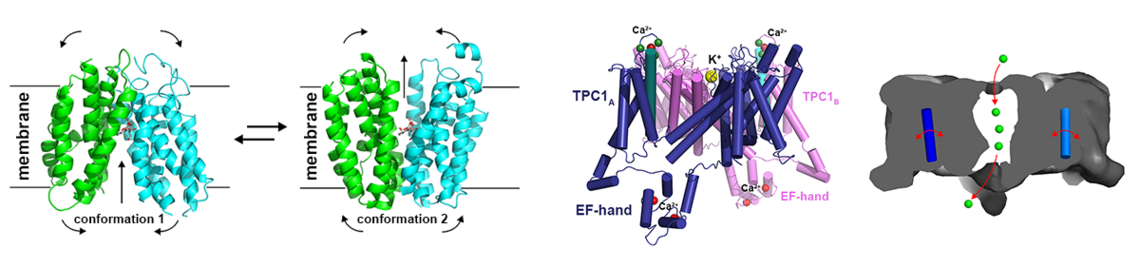
For ion channels that are homologous to membrane proteins with already known structure, 3D models can be prepared by homology modelling and molecular simulations (2-6). These models then may provide predictions about ion selectivity and transport mechanism. However due to the theoretical nature of the models these predictions require stringent testing by mutagenesis and cell assays and/or biophysical analyses. Employing such simulations our group could provide mechanistic insights into the ion transport of different cation channels (2-6).
Maierhofer T, Lind C, Huttl S, Scherzer S, Papenfuss M, Simon J, Al-Rasheid KA, Ache P, Rennenberg H, Hedrich R et al: A Single-Pore Residue Renders the Arabidopsis Root Anion Channel SLAH2 Highly Nitrate Selective. Plant Cell 2014, 26(6):2554-2567.
Bohm J, Scherzer S, Shabala S, Krol E, Neher E, Mueller TD, Hedrich R: Venus Flytrap HKT1-Type Channel Provides for Prey Sodium Uptake into Carnivorous Plant Without Conflicting with Electrical Excitability. Mol Plant 2015.
Scherzer S, Krol E, Kreuzer I, Kruse J, Karl F, von Ruden M, Escalante-Perez M, Muller T, Rennenberg H, Al-Rasheid KA et al: The Dionaea muscipula Ammonium Channel DmAMT1 Provides NH4(+) Uptake Associated with Venus Flytrap's Prey Digestion. Curr Biol 2013, 23(17):1649-1657.
Dadacz-Narloch B, Beyhl D, Larisch C, Lopez-Sanjurjo EJ, Reski R, Kuchitsu K, Muller TD, Becker D, Schonknecht G, Hedrich R: A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 2011, 23(7):2696-2707.
Dunkel M, Latz A, Schumacher K, Muller T, Becker D, Hedrich R: Targeting of vacuolar membrane localized members of the TPK channel family. Mol Plant 2008, 1(6):938-949.
Latz A, Becker D, Hekman M, Muller T, Beyhl D, Marten I, Eing C, Fischer A, Dunkel M, Bertl A et al: TPK1, a Ca(2+)-regulated Arabidopsis vacuole two-pore K(+) channel is activated by 14-3-3 proteins. Plant J 2007, 52(3):449-459.