Anion channels: molecular switches in guard cells
Anion channels: molecular switches in guard cells
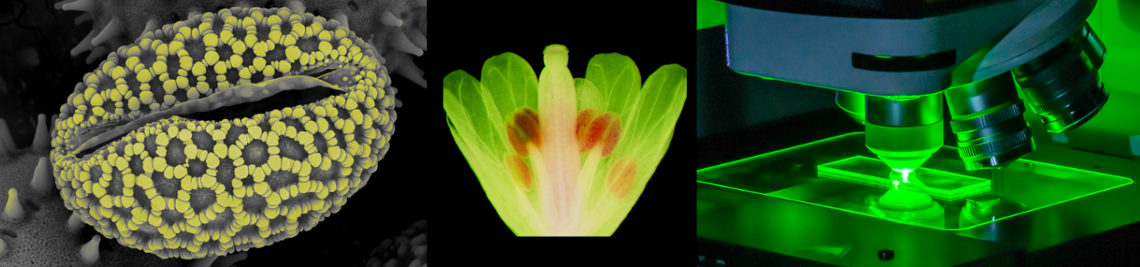
Microscopic stomatal complexes embedded in the epidermis of guard cells control the gas exchange between plants and the atmosphere. These small pores (stoma) are formed between a pair of guard cells and supply the plant with CO2 for photosynthesis. However, CO2 uptake is accompanied by the loss of water via transpiration. To solve this dilemma and to optimize CO2 uptake while minimizing the loss of water, stomata can be opened or closed dependent on a multitude of environmental stimuli such as light, CO2, water availability and pathogen attack plants. The stomatal aperture is controlled by osmotic processes. Via uptake or release of osmotically active ions, guard cells control the stomatal aperture. Thereby the activity of ion channels or transporters are key for a coordinated stomatal opening respectively closing.
The phytohormone ABA plays a crucial role in the response of guard cells to stress conditions. Upon drought e.g., this hormone is synthesized in guard cells and in turn initiates a signalling cascade finally addressing SLAC1-type anion channels. The activity of the anion channels depolarizes the guard cell plasma membrane which in turn open potassium channels. The release of anions together with potassium decreases the guard cell turgor and the stoma closes within a few minutes only.
Within the framework of the CRC/TR166 we could resolve the molecular mechanism of ABA-dependent stomatal closure starting from ABA-perception to SLAC1 anion channel activation. We reconstituted the ABA-signalling cascade in the plant background-free heterologous expression system of Xenopus oocytes and determined the electrical properties of SLAC1 channels with biophysical techniques. Patch clamp and gas exchange experiments with loss-of-function mutants, disturbed in ABA-signalling, confirmed our results obtained in oocytes. Comprehensive evolutionary approaches concerning the co-evolution of stomata and membrane-delimited ABA-signalling finally revealed that during the development of higher plants SLAC1 anion channels co-opted the ancient ABA-signalling pathway. The physiological function of SLAC1 in lower plants such as mosses and ferns remains elusive.
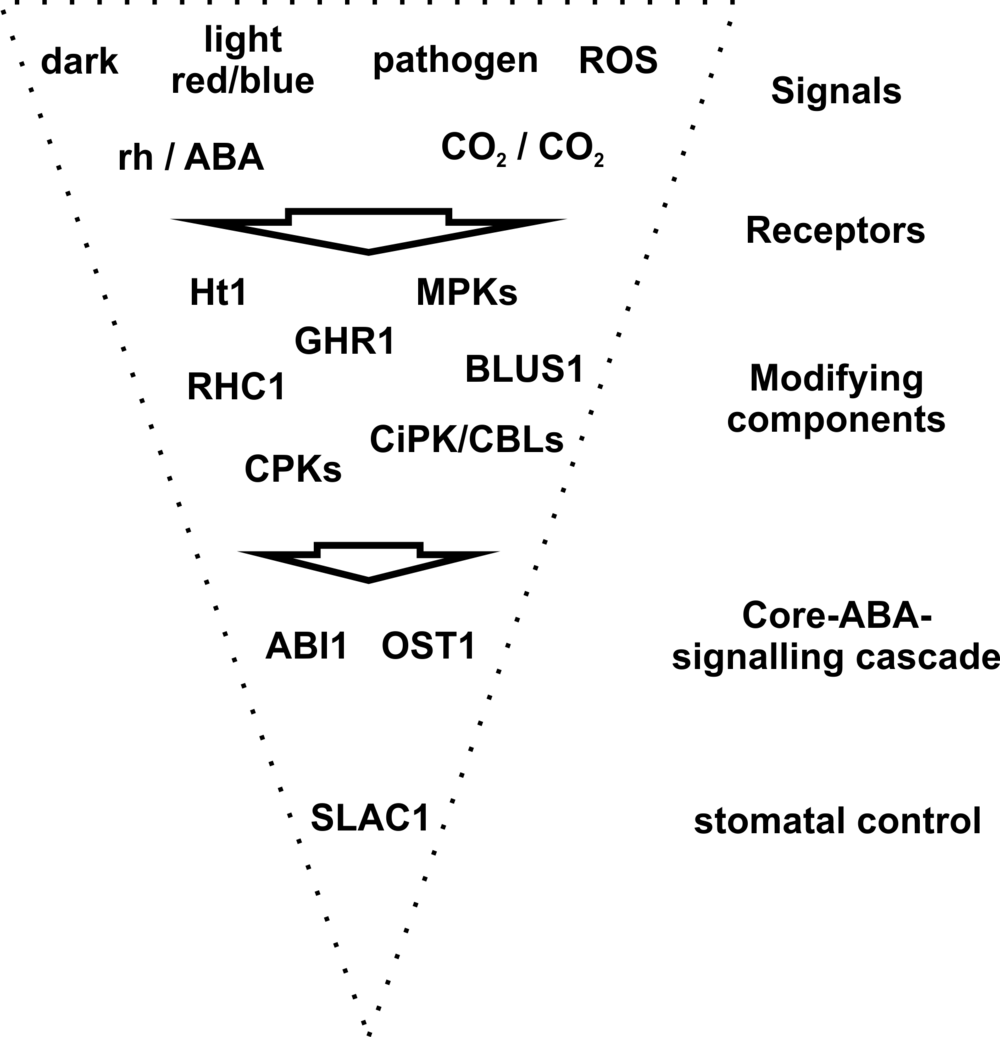
Current experiments in respect to pathogen-, CO2- or rh-induced stomatal closure indicate that the signalling pathways of the different environmental stimuli converge on the core ABA-signalling cascade with SLAC1 representing the final effector protein (Fig. 1). Thus, the multi-sensory guard cells use SLAC1 as molecular switch to react on stress stimuli with fast stomatal closure.