Infection and Cancer
The link between infections and the occurrence of cancer has been firmly established for oncogenic viruses and also for bacteria, with the leading example of Helicobacter pylori as the cause of gastric cancer. In the case of bacterial infection the dominant view has been that it acts mostly indirectly by inducing chronic inflammation that fuels the transformation process. While inflammation certainly contributes to cancerogenesis it has recently been demonstrated that some bacteria also exert severe direct effects on the host cells e.g. by causing DNA damage. While such severe damage ought to promote apoptotic death, several bacteria have been shown to exert pro-survival effects on even damaged cells. This might constitute an alarming situation since infected cells, having acquired genomic alterations, could ultimately survive and evolve towards malignancy.
Numerous additional traits point to a more direct effect of bacterial infections on the initiation and support of tumor growth. Bacteria induce oncogenic signaling and metabolic reprogramming reminiscent of transformed cells in order to support access to nutrients and to prevent their eradication by cell autonomous and innate defense mechanisms. Chronic infections may not only cause long-term chronic inflammation shaping environments supportive for tumor growth, these silent infections may also drive prolonged apoptosis resistance and transformation by subverting host cell signaling. We investigate Chlamydia infection and viral co-infection as a potential risk factor for ovarian and cervix cancer and explore the reprogrammed metabolism as potential target for therapeutic intervention in cancer and infection by intracellular bacteria.
Chlamydia infection – a risk factor for ovarian and cervix carcinoma?
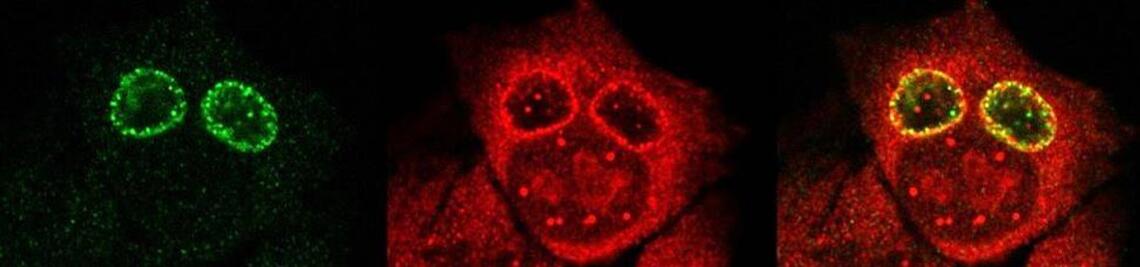
Chlamydia infection induces stress in the host cell that leads to DNA damage. In order to survive these bacteria must downregulate the TP53 tumor suppressor protein that mediates either cell-cycle arrest, suppression of nutrient availability or apoptosis in stressed cells. TP53-dependent pathways are also activated by oncogenes in early tumorigenesis. Evasion of p53-dependent apoptosis is thus a common response of the host to both Chlamydia infection and oncogenic transformation. Strong evidence has accumulated suggesting fallopian tubes, the sites for chronic infections with Chlamydia are the site of origin for ovarian cancer cells and pelvic inflammatory disease (a frequent disease connected to Chlamydia infection) which has been identified as a major risk factor for ovarian cancer. We investigate in this project how chlamydial infection may contribute to the development of ovarian and cervix cancer (read more). We also follow the hypothesis, that Chlamydia – virus co-infections causes genomic instability and increased mutation rates supporting ovarian cancer (read more).