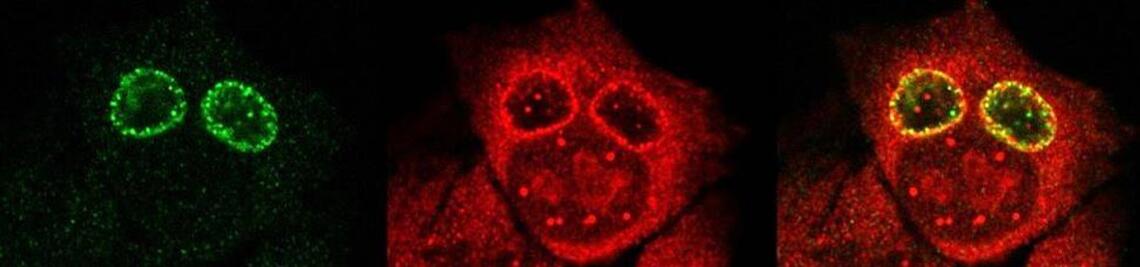
Development of vaccines
We have developed an innovative vaccination strategy against SARS-CoV-2 based on the well-tolerated, oral live Salmonella enterica Serovar Typhi vaccine Ty21a that harbours one of our novel antigen delivery plasmids of the JMU-SalVac-100 series.
The JMU-Salmonella Vaccine (JMU-SalVac) platform technology offers high flexibility and plenty of possible combinations for fine tuning of the desired immune response against various antigens of interest, like for example antigens of SARS-CoV-2.

After the successful completion of her diploma as well as dissertation phase (pathogenicity research) at the Chair of Microbiology under the supervision of Prof. Dr. Werner Goebel, Dr. Birgit Bergmann switched from basic to applied research and initially worked in the field of vaccine development against bacterial infectious agents. Subsequently, she expanded her research area and applied different bacterial carriers also in the therapy of cancer: e.g. Salmonella-based vaccine against prostate cancer or tumor targeting with Listeria as bacterial carrier strains. In her position as a research associate at the University of Würzburg, she was mainly responsible for managing translational research projects conducted in cooperation with partners from the pharmaceutical industry. In addition to research and development, her responsibilities included the design and management of preclinical studies in the context of regulatory approval procedures, e.g. the establishment of test protocols, the coordination of technology transfer to various contract companies as well as the preparation of sample material for toxicity studies including documentation and quality management.
In addition to her scientific expertise, Dr. Bergmann has completed an apprenticeship as a bank clerk as well as an advanced training as a business manager and holds the European Business Competence Licence A and Licence B.
In 2020, she developed the JMU SalVac platform technology together with Prof. Dr. Thomas Rudel. Together with Prof. Rudel, she now heads the vaccine research group at the Department of Microbiology.

As a trained chemical laboratory technician, Heike Czotscher can draw on years of experience in the Department of Microbiology and has worked in a wide range of specialist areas. In this context, she has already successfully carried out various projects with Dr Bergmann. She has been strengthening the JMU SalVac research group since 2021. Here she is primarily responsible for microbiological work. Her core competencies lie in the production of vaccine strains and their in vitro characterization, such as the analysis of antigen expression.

Andrea Fick studied Biology and finished 2006 with a diploma. From 2007 to 2012 she worked as a research associate in the Division of Molecular Internal Medicine in Würzburg. In 2012, she started working at the Chair of Microbiology and has been working there as a technician, with a break of two years during which she worked at the Institute of Virology and Immunology. Her expertise lies with experimental work with mice, genotyping, establishment and performance of ELISAs, as well as in molecular- and cell biology.

Lea Fink is a trained biological-technical assistant. During her training, she gained microbiological as well as immunological experience during an internship at the veterinary office in Karlsruhe and another internship at Rhenser Mineralbrunnen in Rhens. After completing her training in 2022, she joined the JMU SalVac team. As an additional qualification for working with laboratory animals, she completed the FELASA-B course (Federation of European Laboratory Animal Science Associations) and is currently conducting the practical implementation of immunization studies with Salmonella vaccines.

In 2021, Dr. Andreas Kluge completed his Master studies at the Chair of Mikrobiology in the Julius-Maximilians Universität Würzburg, during which he gained practical experience in the field of microbiology in academic and diagnostic-industrial settings in Germany and abroad. Afterwards, he started his PhD Project at the Chair of Microbiology. In the JMU-SalVac Project, his expertise lies with molecular genetics and protein expression of heterologous antigens in Salmonella species, with special focus on the development and optimization of new vaccine candidates.