Host interactions of intracellular Staphylococcus aureus
Staphylococcus aureus causes a variety of diseases ranging from abscesses to endocarditis and sepsis. Treatment options are rapidly dwindling due to the emergence of methicillin-resistant S. aureus (MRSA). During infections, S. aureus is challenged by defensive strategies of the host. For example, the bacteria are taken up by phagocytic immune cells, which usually are able to remove a majority of the bacteria. However, some bacteria may persist in the host either by neutralizing host immune strategies or by hiding inside tissue cells. The latter are able to internalize the pathogen and thus constitute so-called non-professional phagocytes (NPPC). Cytotoxic S. aureus strains often escape from the resulting endocytic vesicles and replicate in the cytoplasm and subsequently cause host cell death. The involved bacterial effectors and host factors in this complex interplay and the underlying molecular mechanisms are largely unknown.
The research group uses fluorescent reporters in both, host cells and the pathogen, to monitor and analyze the infection dynamics. Further, the group employs transposon mutant screens coupled to next-generation sequencing techniques to identify bacterial virulence factors required for establishment of infection in specific intracellular and extracellular infection niches.
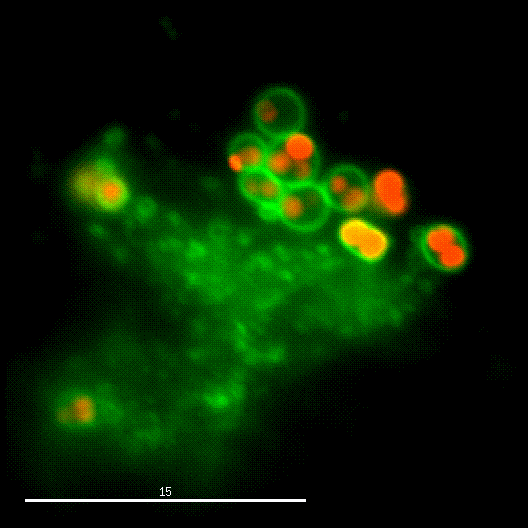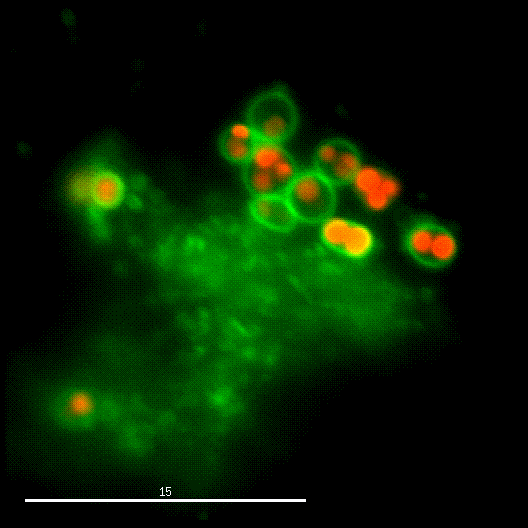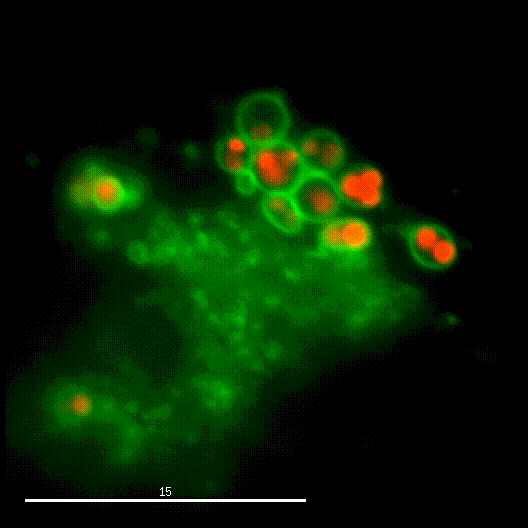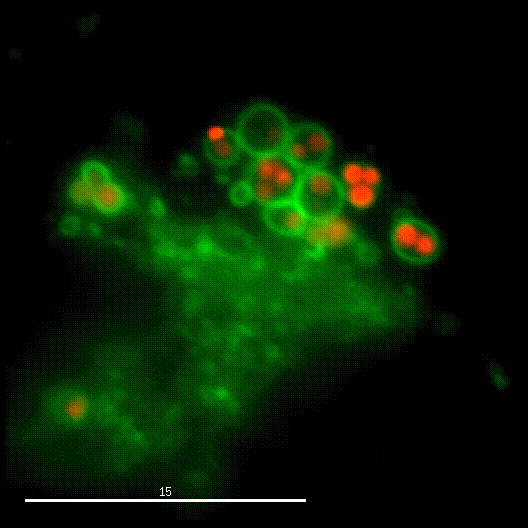
One major research focus of the group currently is the interaction of S. aureus with sphingolipids on the host cell surface, the resulting bacterial uptake, and the intracellular fate of the bacteria. S. aureus produces a variety of plasma membrane-active toxins, as wells as a bacterial sphingomyelinase and various other virulence factors, which modulate or permeabilize cellular membranes at the host cell surface or within endosomes. The group focuses on altered bacterail virulence upon changes in sphingolipid content of host membranes.
Together with Prof. Dr. Christian Stigloher (Imaging Core Facility) we want to understand S. aureus infections on host membranes and their dependencies on sphingolipids on an organismal level for which Prof. Stigloher has established the genetically tractable model organism C. elegans as an infection model for S. aureus.
Due to the fruitful cooperation with the organic Chemistry Department of Prof. Dr. Jürgen Seibel and the Bioimaging department of Prof. Dr. Markus Sauer, both members of the RTG2581 consortium, we now can use modified or functionalized sphingolipids to visualize S. aureus-infected cells and the respective host membranes with Expansion Microscopy (ExM) at high spatial resolution with standard confocal microscopy equipment (see also below).
The funding for this project is provided by the Deutsche Forschungsgemeinschaft (DFG) within the Research Training Group RTG2581 “SPHINGOINF”.
Another major focus of the laboratory is the dissemination of the pathogen in the host.
Together with Prof. Dr. Eva Media at the HZI Braunschweig we investigate so called Molecular Decision Points underlying the systemic spreading of Staphylococcus aureus through the infected host organism. This collaborative research is based on the observation that S. aureus is able to disseminate via the bloodstream of the host from an initial local site of infection to distant organs. Thereby the pathogen can cause progressive, chronic, or recurrent infections. When S. aureus is in the bloodstream it initially passes through the liver, which constitutes the first line of defence.Liver-resident macrophages, so-called Kupffer cells, thereby filter the bacteria out of the circulation.
When blood stream phagocytes and Kupffer cells fail to eliminate the pathogen completely, the bacteria can disseminate to secondary organs, such as kidneys and bones where cells of the host immune system wall off the bacteria by abscess formation.
However, also abscesses fail to contain S. aureus completely which may result in recurrence of infection. We here want to identify critical host factors, the so-called Molecular Decision Points which control the capacity of phagocytes to clear S. aureus from the bloodstream. Within our project C04 in the Collaborative Research Center DECIDE we have the ideal research conditions to investigate this important field of S. aureus infection biology.
Some of our previous findings
A Staphylococcus aureus LysR-Type Transcriptional Regulator is crucial for secondary tissue colonization during metastatic bloodstream Infection
Together with Eva Medina (Braunschweig), we recently identified a novel LysR-type transcriptional regulator in S. aureus, which is required to establish staphylococcal infections specifically in kidneys or bones of the host but was dispensable for liver infections. For identification we employed an unbiased transposon mutant pool screens in vivo followed by transposon insertion site deep sequencing (TnSeq). Since this transcription factor previously was not found to be produced under a variety of tested conditions, its regulon was unknown. We therefore induced its production and sequenced the transcriptome by RNA-seq. Promoter activity reporters further demonstrated, that the transcription factor is repressed by glucose and requires copper ions for activity. Our results thereby hint at differences in metabolic adaptation of the pathogen in its infection niches in the host. Thus, by analyzing S. aureus, we can learn about specific conditions within the host.
Ref. :
- Groma M, Horst SA, Das S, Huettel B, Klepsch M, Rudel T, Medina E, Fraunholz M (2020) Identification of a Novel LysR-Type Transcriptional Regulator in Staphylococcus aureus That Is Crucial for Secondary Tissue Colonization during Metastatic Bloodstream Infection. mBio 11(4):e01646-20
The Staphylococcus aureus AraC-Familiy Transcriptional Regulator Rsp is crucial for cytotoxicity and natural mutations in the respective gene cause a change from acute to persistent infections
Already earlier, we had used a similar TnSeq strategy in lung infection models and thus identified another staphylococcal transcription factor, Rsp, to be required for full virulence in pneumonia. We found that S. aureus rsp mutants had reduced cytotoxicity and were avirulent in lung infections. Identification of the transcription factor regulon established the long non-coding RNA SSR42 (lncRNA) to be transcribed by Rsp. Mutants within the genes for Rsp or SSR42 lost their hemolytic and cytotoxic phenotypes. Most interestingly, S. aureus rsp mutants can be recovered from bacteremia isolates in patients with nasal S. aureus carriage. This demonstrated that during human nasal carriage S. aureus is able to acquire mutations within the rsp locus, which can influence the S. aureus pathotype. The function of theRsp-regulated lncRNA is still enigmatic.
Refs.:
- Das S, Lindemann C, Young BC, Muller J, Österreich B, Ternette N, Winkler AC, Paprotka K, Reinhardt R, Förstner KU, Allen E, Flaxman A, Yamaguchi Y, Rollier CS, van Diemen P, Blättner S, Remmele CW, Selle M, Dittrich M, Mueller T, Vogel J, Ohlsen K, Crook DW, Massey R, Wilson DJ, Rudel R, Wyllie DH, Fraunholz MJ (2016) Natural mutations in a Staphylococcus aureus virulence regulator attenuate cytotoxicity but permit bacteremia and abscess formation. PNAS 113:E3101-10
- Horn J, Klepsch M, Manger M, Wolz C, Rudel T, Fraunholz M (2018) Long Noncoding RNA SSR42 Controls Staphylococcus aureus Alpha-Toxin Transcription in Response to Environmental Stimuli. Journal of Bacteriology 200(22)
Phenol-soluble modulins as well as a non-ribosomal peptide synthase of Staphylococcus aureus are involved in phagosomal escape of the pathogen
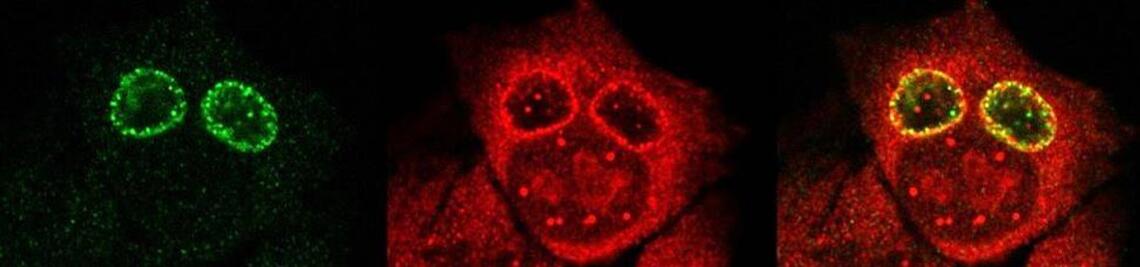
In order to investigate staphylococcal factors required for phagosomal escape, we developed escape reporter-expressing cells, which we infected with a variety of S. aureus mutant strains in order to measure the escape proficiency of each strain by automated microscopy. We thereby identified candidate bacterial genes encoding a non-ribosomal peptide synthase (NRPS). The S. aureus NRPS and its peptide product, the cyclic dipeptide phevalin, enhance S. aureus phagosomal escape thereby contributing to epithelial and immune cell death. Bacterial mutants in the NRPS genes were significantly attenuated in vitro and also in vivo. Thus, the NRPS AusAB (PznAB) next to the phenol soluble modulins (PSM) constitutes a second factor required by S. aureus to translocate from the endomembrane vesicles of NPPC to the host cytoplasm.
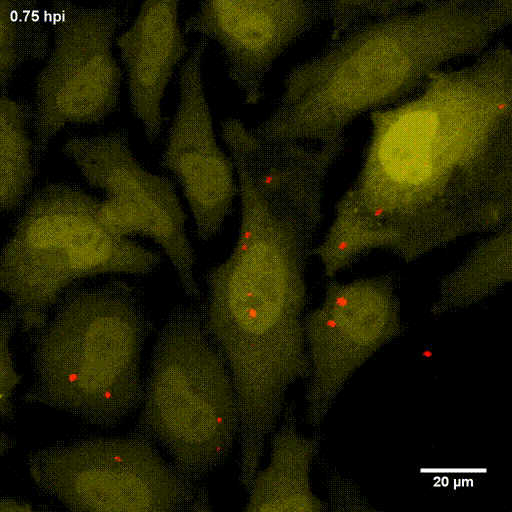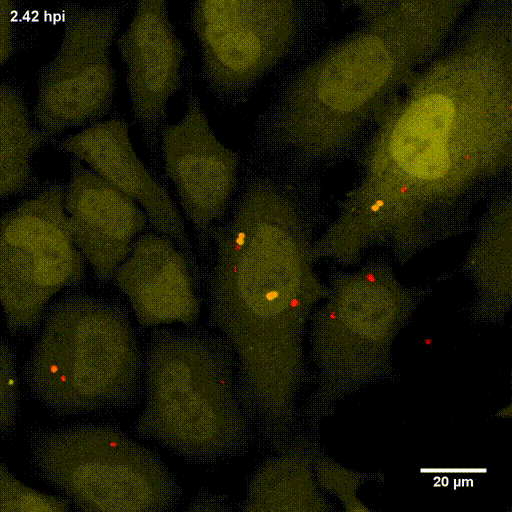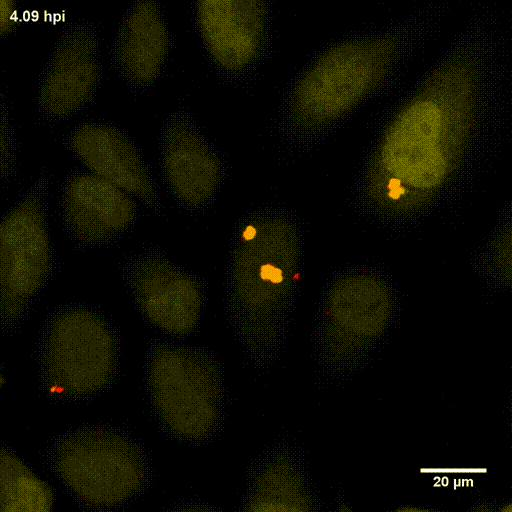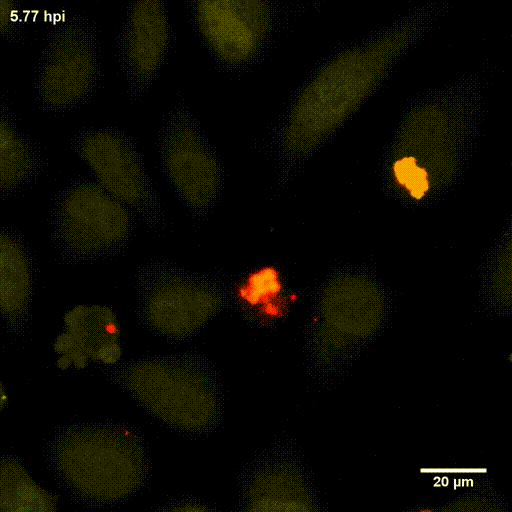
Ref.:
- Blättner S, Das S, Paprotka K, Eilers U, Krischke M, Kretschmer D, Remmele CW, Dittrich M, Müller T, Schuelein-Voelk C, Hertlein T, Mueller MJ, Huettel B, Reinhardt R, Ohlsen K, Rudel T, Fraunholz MJ (2016) Staphylococcus aureus Exploits a Non-ribosomal Cyclic Dipeptide to Modulate Survival within Epithelial Cells and Phagocytes. PLoS Pathogens 12:e1005857
- Grosz, M., Kolter, J., Paprotka, K., Winkler, A.C., Schafer, D., Chatterjee, S.S., Geiger, T., Wolz, C., Ohlsen, K., Otto, M., Rudel, T., Sinha, B. and Fraunholz, M. (2014). Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin alpha. Cell Microbiol 16, 451-465.
Host cell death caused by intracellular Staphylococcus aureus is influenced by calcium levels and the bacterial cysteine protease Staphopain A
Once in the host cytoplasm, S. aureus perturbs intracellular calcium levels, replicates (YouTube video by Dr. Kathrin Stelzner https://www.youtube.com/watch?v=OqCVZ_S10rw) and shortly thereafter infected host cells die. Together with the group of Thomas Rudel, we demonstrated that host cell death of cytoplasmic S. aureus requires the bacterial cysteine protease staphopain A (ScpA), however the targets of which in the host cytoplasm currently are unknown.
Refs.:
- Stelzner K, Winkler AC, Liang C, Boyny A, Ade CP, Dandekar T, Fraunholz MJ, Rudel T (2020) Intracellular Staphylococcus aureus Perturbs the Host Cell Ca2+ Homeostasis To Promote Cell Death. mBio 11(6):e02250-20
- Stelzner K, Boyny A, Hertlein T, Sroka A, Moldovan A, Paprotka K, Kessie D, Mehling H, Potempa J, Ohlsen K, Fraunholz MJ, Rudel T (2021) Intracellular Staphylococcus aureus employs the cysteine protease staphopain A to induce host cell death in epithelial cells. PLoS Pathogens 17(9):e1009874.
Host plasma membrane permeabilization by staphylococcal α-toxin results in activation of sphingomyelinase dependent membrane repair mechanisms
Extracellular S. aureus further uses one of its pore-forming toxins, α-toxin, to induce calcium influx into host cytoplasm. Permeabilized host cell membranes are repaired by a mechanism involving endocyctosis of damaged membranes, as well as recruitment of membrane repair enzymes such as acid sphingomyelinase (ASM), which is located in host lysosomes. Thus, the host cell uses a conserved repair mechanism already described for other toxins to reseal membranes damaged by staphylococcal α-toxin. We currently investigate the contribution of host sphingolipids to S. aureus cellular microbiology within GRK2581 SPHINGOINF.
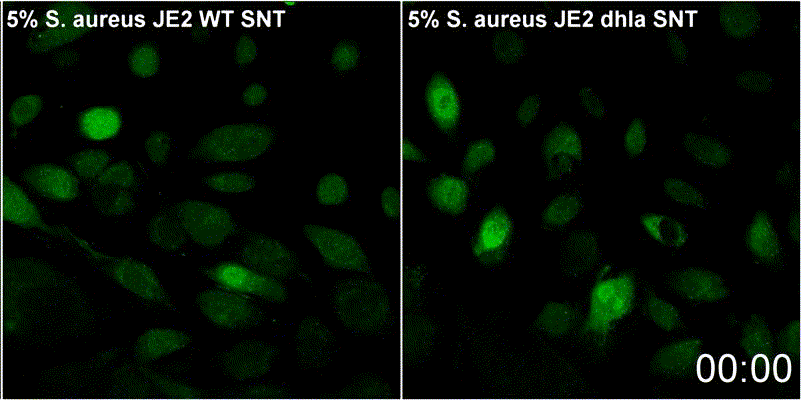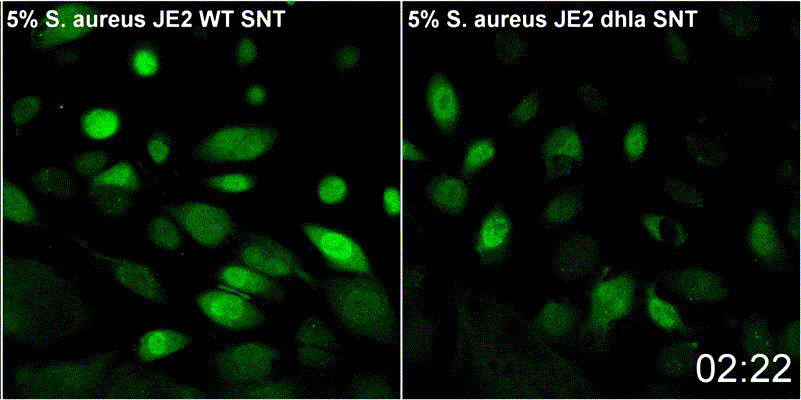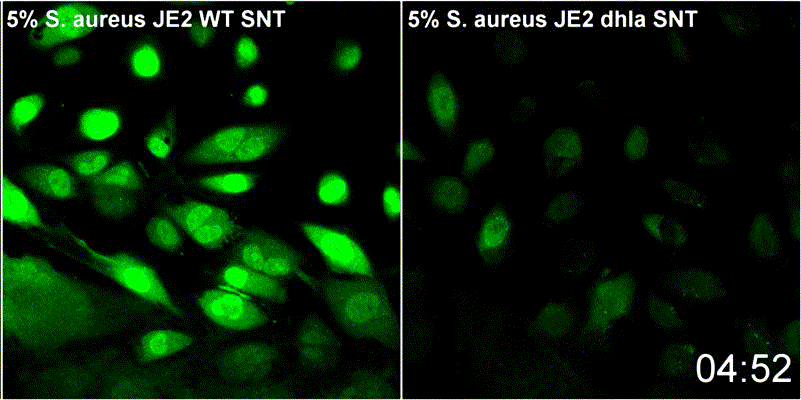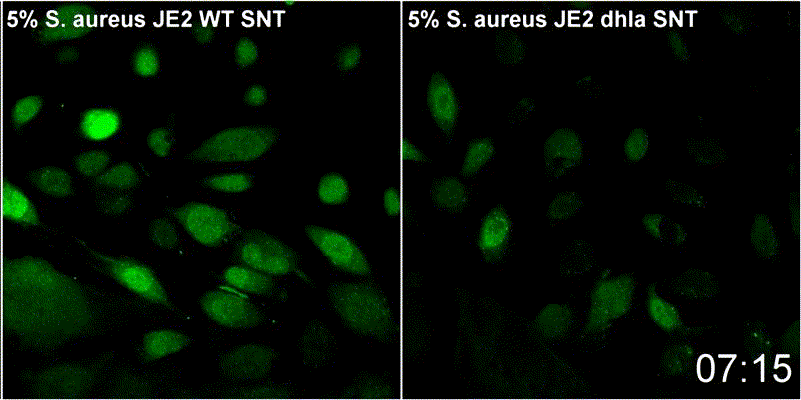
Ref.:
- Krones D, Rühling M, Becker KA, Kunz TC, Sehl C, Paprotka K, Gulbins E, Fraunholz M (2021) Staphylococcus aureus α-Toxin Induces Acid Sphingomyelinase Release From a Human Endothelial Cell Line. Frontiers in Microbiology 12:694489
Expansion Microscopy of Staphylococcus aureus requires the use of a specific protease cocktail
Since many of the groups research questions require fluorescence microscopy, we investigated, if so-called Expansion microscopy (ExM) may be applicable to S. aureus-infected cells. ExM circumvents the diffraction limitation of classical microscopy techniques by swelling the sample. Therein, the rigidity of the staphylococcal cell wall was overcome by application of specific proteases and now enables us to image host-pathogen interactions with at least four-fold higher resolution with standard confocal fluorescence microscopes. This work was possible due to the collaborations within the GRK2581 SPINGOINF (see above).
Ref:
- Kunz TC, Rühling M, Moldovan A, Paprotka K, Kozjak-Pavlovic V, Rudel T, Fraunholz M (2021) The Expandables: Cracking the Staphylococcal Cell Wall for Expansion Microscopy. Frontiers in Cellular and Infection Microbiology 11:644750
Associations
We are proud to be associated with the DFG priority program SPP2225 EXIT, which is a consortium of researchers that investigate exit strategies of intracellular pathogens. SPP2225 thereby explores convergently evolved exit pathways employed by bacterial, parasitic and fungal pathogens with relevance for human health.
Material Availability
Our improved host cell escape reporter YFP-CWT (https://www.addgene.org/176689/) as well as the brilliantly fluorescent proteins Cerulean (https://www.addgene.org/24756/) and mRFP (https://www.addgene.org/26252/) for expression in S. aureus are available via AddGene.