benavente lab
We investigate the molecular organization, function and evolutionary history of the mammalian meiotic chromosome
Research synopsis
Selected publications
RESEARCH TOPICS
Molecular Architecture of the Meiotic Chromosome
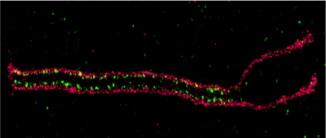
Evolutionary History of Meiosis
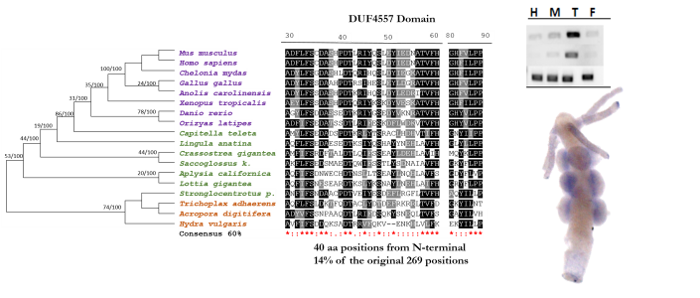
Meiotic Gene Expression

A collaborative project with colleagues of the Clemente Estable Institute for Biological Research (Uruguay) to elucidate stage-specific gene expression of meiotic cells
Architecture and Function of the Nuclear Envelope
Roberta Sciurano

Visiting scientist
Fernanda Trovero

Visiting scientist