Epigenetic genome reprogramming: developmental origins of health and disease
Lab Members:
Dr. Ramya Potabattula, PhD
Laura Bernhardt, MSc
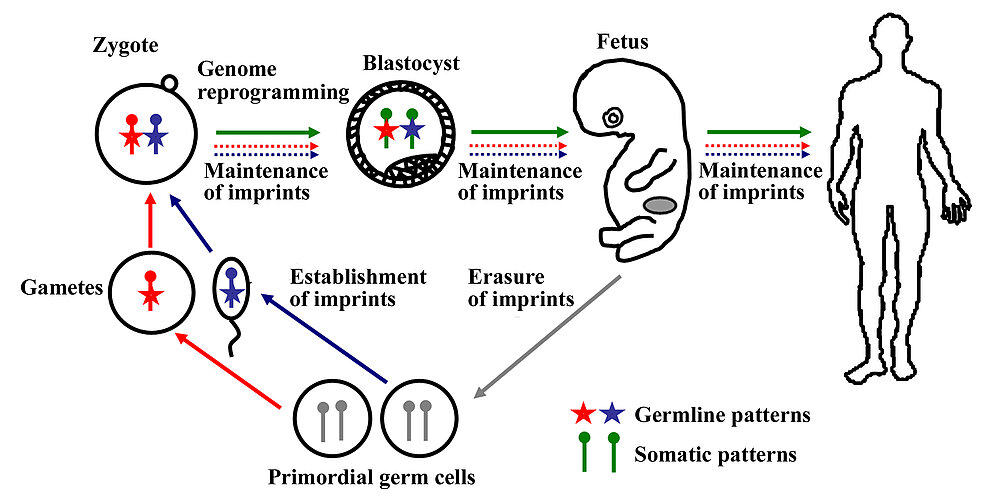
Epigenetic information is not encoded by the DNA sequence itself but by reversible modifications of DNA and/or histones. Epigenetic mechanisms control the spatial, temporal and parent-specific gene expression. Different cell types are endowed with different methylation patterns of silenced and expressed genes, which are set during development and differentiation and then stably inherited during cell divisions. In mammals, the paternal and maternal genomes undergo two rounds of parent-specific DNA methylation reprogramming (Fig. 1). Stochastically, genetically and/or environmentally induced errors (epimutations) in this highly coordinated process may contribute to human disease. The first round of genome-wide demethylation and remethylation occurs in the germline. When the primordial germ cells enter the fetal gonadal ridge, all DNA methylation patterns of the previous generation(s) are erased, restoring totipotency and an equivalent epigenetic state in germ cells of both sexes. Sex-specific methylation patterns are then established during male versus female germ-cell differentiation. In the second round of genome reprogramming after fertilization the methylation patterns for totipotency and somatic development must be restored. Genome-wide demethylation in the zygote and early embryo occurs in a parent-specific manner, broadly affecting different classes of repetitive and single copy sequences. Only imprinted genes are protected by an unknown mechanism against this postzygotic demethylation, maintaining their germline methylation marks and parent-specific activities throughout further development. De novo methylation occurs preferentially in the inner cell mass of blastocyst embryos, establishing somatic methylation patterns in the precursor cells of the different embryonic lineages.
Potabattula R., Dittrich M., Böck J., Haertle L., Müller T., Hahn T., Schorsch M., El Hajj N., Haaf T. Allele-specific methylation of imprinted genes in fetal cord blood is influenced by cis-acting genetic variants and parental factors. Epigenomics 10, 1315-1326 (2018).
Haaf T. Methylation dynamics in the early mammalian embryo: implications of genome reprogramming defects for development. Curr. Top. Microbiol. Immunol. 310, 13-22 (2006).
Mayer W., Niveleau A., Walter J., Fundele R., Haaf T. Demethylation of the zygotic paternal genome. Nature 403, 501-502 (2000).
Animal experiments and epidemiological studies in humans suggest that adverse (i.e. periconceptional and intrauterine) exposures during early development can be associated with negative health outcomes later in life, in particular increased rates for many metabolic and cardiovascular diseases. The developmental origins of health and disease (DOHaD) hypothesis proposes that prenatal environmental factors have long-lasting effects on the setting of metabolic, neuroendocrine and other pathways and, thus, influence disease susceptibilities in later life. Epigenetic changes induced by environmental factors are thought to stably modulate gene expression, providing a link between early life conditions and susceptibility to complex disease.
El Hajj N., Schneider E., Lehnen H., Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 148, R111 R120 (2014).
Lehnen H., Zechner U., Haaf T. Epigenetics of gestational diabetes mellitus and offspring health: the time for action is in early stages of life. Mol. Hum. Reprod. 19, 415 422 (2013).
Although assisted reproductive technologies (ARTs) have become a routine practice for human infertility treatment, the etiology of the increased risks for medical problems in ART-conceived children is still poorly understood (Fig. 2). ARTs involve dramatic environmental changes during late oocyte development and early embryogenesis. Our group analyses the epigenetic effects of different ARTs on the germ cells and offspring health.
von Wolff M, Haaf T. In vitro fertilization technology and child health. Risks, mechanisms and possible consequences. Dtsch. Ärztebl. Int. 117, 23-30 (2020).
El Hajj N., Haertle L., Dittrich M., Denk S., Lehnen H., Hahn T., Schorsch M., Haaf T. DNA methylation signatures in cord blood of IVF/ICSI children. Hum. Reprod. 32, 1761-1769 (2017).
Heinzmann J., Mattern F., Aldag P., Bernal-Ulloa S.M., Schneider T., Haaf T., Niemann H. Extended in vitro maturation affects gene expression and DNA methylation in bovine oocytes. Mol. Hum. Reprod. 21, 770-782 (2015).
Pliushch M., Schneider E., Schneider T., El Hajj N., Rösner S., Strowitzki T., Haaf T. In vitro oocyte maturation is not associated with altered deoxyribonucleic acid methylation patterns in children from in vitro fertilization or intracytoplasmatic sperm injection. Fertil. Steril. 103, 720-727 (2015).
El Hajj N., Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil. Steril. 99, 632-641 (2013).
The prevalence of diabetes and obesity is increasing in most populations worldwide. In addition to overnutrition and physical inactivity, prenatal factors appear to make a significant contribution to the metabolic disease epidemic. It is well known that the offspring of diabetic and/or obese mothers who are exposed to high concentrations of nutrients in utero are at increased lifelong risk of developing metabolic disorders. We study the epigenetic effects of gestational diabetes which affect up to 10% of all pregnancies in developed countries, and other intrauterine exposures.
De Jesus D.F., Orime K., Kaminska D.,…, Haaf T., Pihlajamäki J., Kulkarni R.N. Parental metabolic syndrome epigenetically reprograms offspring hepatic lipid metabolism in mice. J. Clin. Invest. 130, 2391-2407 (2020).
Mattern F., Post A., Solger F., O'Leary A., Slattery D., Reif A., Haaf T. Prenatal and postnatal experiences associated with epigenetic changes in the adult mouse brain. Behav. Brain Res. 359, 143-148 (2019).
Mitchell M., Strick R., Strissel P.L., Dittrich R., McPherson N.O., Lane M., Pliushch G., Potabattula R., Haaf T., El Hajj N. Gene expression and epigenetic aberrations in F1-placentas fathered by obese males. Mol Reprod. Dev. 84, 316-328 (2017).
Haertle L., El Hajj N., Dittrich M., Mueller T., Nanda I., Lehnen H., Haaf T. Epigenetic signatures of gestational diabetes mellitus on cord blood methylation. Clin. Epigenet. 9, 28 (2017).
El Hajj N., Pliushch G., Schneider E., Dittrich M., Müller T., Korenkov M., Aretz M., Zechner U., Lehnen H., Haaf T. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes 62, 1320-1328 (2013).
Accumulating evidence suggests that paternal factors can also confer increased disease risks for the next generation. Our group has a long-standing interest into the effects of advanced paternal age and BMI on the sperm epigenome and the resulting embryos/offspring.
Potabattula R., Zacchini F., Ptak G.E., Dittrich M., Müller T., El Hajj N., Hahn T., Drummer C., Behr R., Lucas-Hahn A., Niemann H., Schorsch M., Haaf T. Increasing methylation of sperm rDNA and other repetitive elements in the aging male mammalian germline. Aging Cell 19, e13181 (2020).
Potabatulla R., Dittrich M., Schorsch M., Hahn T., Haaf T., El Hajj N. Male obesity effects on sperm and next generation cord blood DNA methylation. PLoS ONE 14:e0218615 (2019).
Atsem S., Reichenbach J., Potabatulla R., Dittrich M., Nava C., Depienne C., Boehm L., Rost S., Hahn T., Schorsch M., Haaf T., El Hajj N. Paternal age effects on sperm FOXK1 and KCNA7 methylation and transmission into the next generation. Hum. Mol. Genet. 25, 4996-5005 (2016).
Kuhtz J., Schneider E., El Hajj N., Zimmermann L., Fust O., Linek B., Seufert R., Hahn T., Schorsch M., Haaf T. Epigenetic heterogeneity of developmentally important genes in human sperm: implications for assisted reproduction outcome. Epigenetics 9, 1648-1658 (2014).
El Hajj N., Zechner U., Schneider E., Tresch A., Gromoll J., Hahn T., Schorsch M., Haaf T. Methylation status of imprinted genes and repetitive elements in sperm DNA from infertile males. Sex. Dev. 5, 60-69 (2011).