Projects
The role of anion channels in polarity control of tip growing plant cells
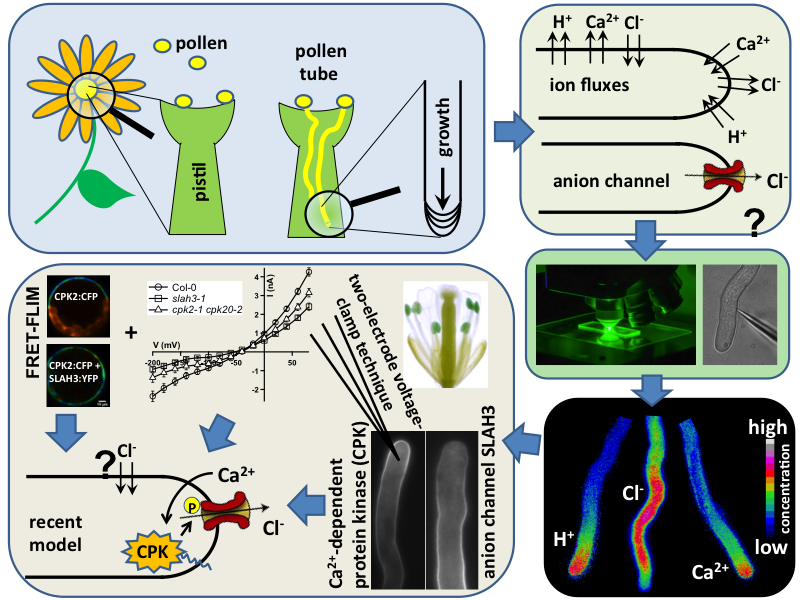
Root hairs and pollen tubes extend by rapid elongation that occurs exclusively at the tip. Fundamental for this local, tip-focused growth (so-called ‘tip growth’) is the polarization of the cytoplasm and the presence of internal ionic gradients. These ionic gradients generated by distinct zones extruding protons, potassium and/or anions as well as zones characterized by calcium- and/or K+ influx create an external electrical field. When this electric field is superimposed by an external electromagnetic field or exposed to ionic gradients across the cell, growth direction changes.
The final goal of this project is to unravel the molecular nature of the ion conductance required to establish the endogenous electrical field and in turn growth direction. This will be realized mainly by studying root hair and pollen tube growth in Arabidopsis mutants lacking the anion channels SLAH2, SLAH3 and ALMT12 very recently identified in our lab. To monitor and quantify polar growth of the mutants, we map anion- as well as cytoplasmic calcium concentrations spectroscopically and measure ionic currents along both expanding cell types electrophysiologically. Finally data on ion channel-dependent polar growth will be used to gain a testable working model for pollen tube/root hair action.
Various „state-of-the-art“-techniques are used to investigate the relationship between intracellular ion concentrations, extracellular ion fluxes and ion channel activity to generate the endogenous electric field. Within the framework of this project, a combination of different techniques like molecular-biology, spectroscopy and electrophysiology complement each other perfectly. The techniques used are for example biolistic transformation, the Two-Electrode-Voltage-Clamp (TEVC) technique with polar growing plant cells and oocytes of the clawed frog (Xenopus laevis), live-cell fluorescence resonance energy transfer (FRET)- imaging, FRET-FLIM (Fluorescence Lifetime Imaging Microscopy) or microelectrode ion flux measurements (MIFE) also known as vibrating probe (VP)- method.
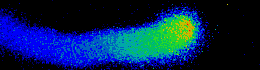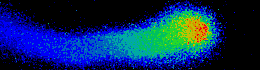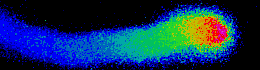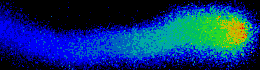
Illustrated are the separate steps, from tasks and aims of the project, to the methods applied and results achieved. Pollen tubes are formed by germination of pollen upon landing on the stigma of all flowering plants. Their reproductive function is to carry the gametes through the pistil, up until the embryo sac in order to deliver the two sperm cells for double fertilization. Anion fluxes are associated with the polar growth process of pollen tubes, but their physiological role is unknown. A combination of molecular biology-, electrophysiological- and spectroscopic techniques was used to identify pollen tube anion channels. The cytosolic anion concentration was measured in growing pollen tubes and anion channel activity was analyzed in anion channel- and CPK loss-of-function mutant background. Within the framework of the project the subcellular localization and identity of the anion channel(s) and their modulating interacting kinases (CPKs) could be resolved. Our studies provide evidence for a Ca2+-dependent CPK2/CPK20 regulation of the anion channel SLAH3 to promote pollen tube growth. Results of this project have recently been published in The Plant Cell (Gutermuth et al., 2013).