MEMBRANE BIOPHYSICS
Cell models and model cells
shed light on processes as at the cellular interface with the outside.
VSG diffusion on the surface
of trypanosomes is reminiscent of the narrow escape problem.
Signaling and mobility
of the G protein-coupled receptor CXCR4 are strongly correlated.
Biomembranes
Membranes are an integral component of every cell. Their main function is compartmentalization. They enclose the organells within a cell as well as separate cells from their surrounding. Next to this barrier function membranes play a crucial role in a multitude of associated processes, e.g., transport, signaling, and adhesion. There is constant exchange of material and information, but in a highly regulated way. As the plasma membrane is at the interface of one cell with another, membrane processes also play an important role in adhesion to the extracellular matrix or other cells.
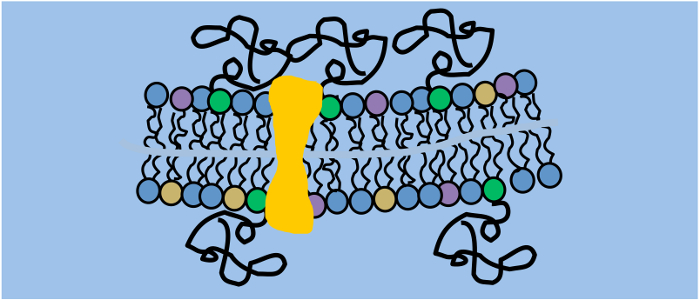
The plasma membrane consists of a lipid double layer, that is not homogeneous, but asymmetric and contains many different lipid types as well as nanometric, dynamic domains enriched in cholesterol and sphingolipids. There are various associated proteins, which can be transmembrane or anchored to the inner or outer leaflet. At the outside, a dense hydrocarbon mesh originating from glycolipids and glycoproteins form the glycocalyx. Next to the cytoplasmic leaflet is the crowded cytosol including the cytoskeleton network. As it is quite challenging to quantitatively investigate one isolated process in such a complex environment, we work in model membranes whenever it is feasible. Next to the pure model systems, giant unilamellar vesicle (GUV) and solid-supported lipid bilayer (SLB) consisting of synthetic phospholipids, also advanced cell models are developed that e.g. allow proper reconstitution of transmembrane proteins.
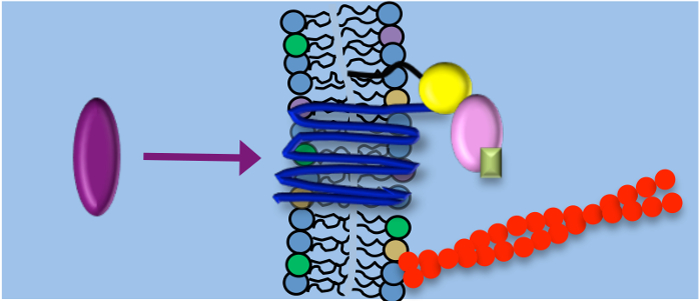
Membrane proteins depend on a membrane environment to fold and function correctly. Next to the traditional detergent-based approaches, reconstitution of transmembrane proteins in cell-sized proteoliposomes can be achieved by a combination of cell-free protein expression and electroformation. Using an eukaryotic protein expression systems ensures that the necessary posttranslational modifications are carried out. Various fluorescent protein-labeled membrane proteins were successfully incorporated into GUVs (e.g. ETB or CXCR4) using our approach.
Signaling and shielding
Using single-molecule fluorescence microscopy we investigate different membrane functions. Two examples include signaling through the chemokine receptor CXCR4 and the shielding function of the variant surface glycoprotein (VSG) coat of African trypanosomes.
CXCR4 is a key player in physiological and pathological processes, e.g. during chemotaxis and metastasis. In order to elucidate the regulation of CXCR4 signaling, we monitor the diffusion behavior of CXCR4 receptors in the resting state and upon stimulation with its chemokine SDF as well as after application of specific drugs to identify interaction partners. We found that activation of the receptors did not change the monomeric state of CXCR4, but induced immobilization of the initially freely diffusing receptor. Immobilization was attributed to two effects, (i) association with endocytotic clathrin coated pits (desensitization) and (ii) Gai mediated supramolecular scaffolding (signal enhancement). Thus, a balanced regulation of G-protein-dependent and independent pathways simultaneously control CXCR4 mobility and signal transduction.
The VSG coat of trypanosomes is crucial for the parasites’ survival in the bloodstream of their host. We performed ensemble and single-molecule microscopy both in vivo and in vitro to show that the density and height of the coat was optimized to ensure at the same time maximum shielding and fluidity. Coat fluidity has to be rigorously maintained as exo- and endocytosis are restricted to a small membrane invagination at the base of the flagellum, the so-called flagellar pocket (FP). This scenario is reminiscent of the narrow escape problem (NEP) dealing with Brownian particles confined to a given domain with reflecting borders and only a small opening where the particles are absorbed. However, we find a clear discrepancy between the theoretical computed time of coat exchange through the FP and the experimental result. We search for an explanation, e.g., the presence of directed transport, by studying the NEP in vivo in trypanosomes as well as in vitro in micro-structured solid-supported lipid bilayers.
Selected publications
Glogger M., Subota I., Pezzarossa A., Denecke A.-L., Carrington M., Fenz S.F., Engstler M. (2017) Facilitating trypanosome imaging, Experimental Parasitology, doi: 10.1016/ j.exppara.2017.03.010.
Glogger M, Stichler S, Subota I, Bertlein S, Spindler M-C, Tessmar J, Groll J, Engstler M, Fenz S (2017) Live-cell super-resolution imaging of intrinsically fast moving flagellates, Journal of Physics D: Applied Physics, doi: 10.1088/1361-6463/aa54eb
Hartel AJW, Glogger M, Jones NG, Abuillan W, Batram C, Hermann A, Fenz SF, Tanaka M, Engstler M (2016) N-glycosylation enables high lateral mobility of GPI-anchored proteins at a molecular crowding threshold. Nature Communications, doi: 10.1038/ncomms12870.
Hartel AJW, Glogger M, Guigas G, Jones NG, Fenz S, Weiss M, Engstler M (2015) The molecular size of the extra-membrane domain influences the diffusion of the GPI-anchored VSG on the trypanosome plasma membrane, Scientific Reports, doi: 10.1038/srep10394
Beletkaia L, Fenz S, Pomp W, Snaar-Jagalska BE, Hogendoorn PWC, Schmidt T (2016) CXCR4 signaling is controlled by immobilization at the plasma membrane, BBA: Molecular Cell Research, doi: 10.1016/j.bbamcr.2015.12.020
Sachse R, Dondapati SK, Fenz S, Schmidt T, Kubick S (2014) Membrane protein synthesis in cell-free systems: From bio-mimetic systems to bio-membranes, Febs Letters, doi: 10.1016/j.febslet.2014.06.007
Fenz S, Sachse R, Schmidt T, Kubick S (2014) Cell-Free Synthesis of MembraneProteins: Tailored Cell Models out of Microsomes, BBA: Biomembranes, doi: 10.1016/j.bbamem.2013.12.009