TRAFFICKING OF GPI-PROTEINS
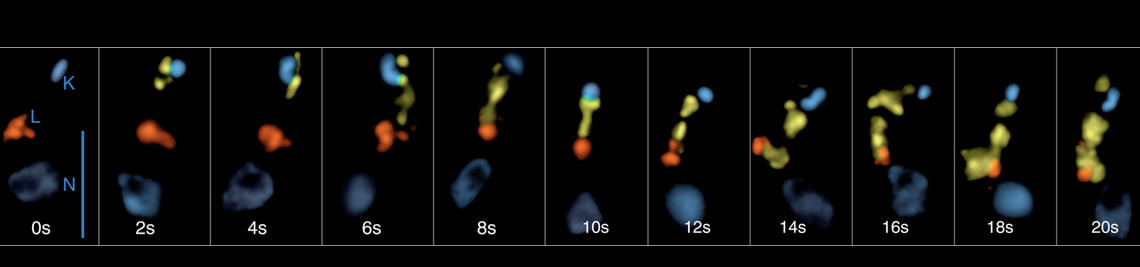
Trafficking of GPI-Proteins (Engstler lab)
Embedded in the outer membrane leaflet
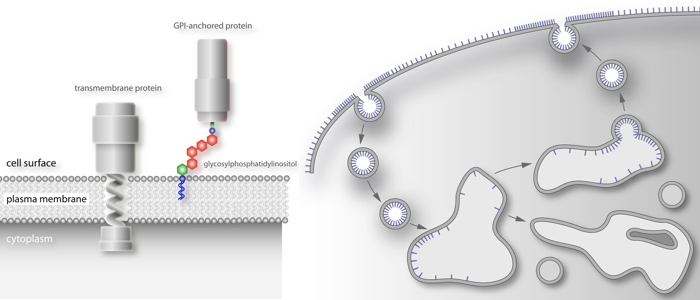
and thus, very different from transmembrane proteins.
GPI = glycosylphoshatidylinositol
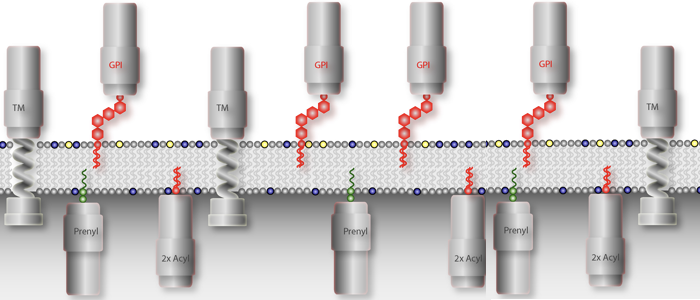
is basically a fatty acid chain with sugars.
How are GPI-proteins sorted?
Still a matter of debate.
A universal building block
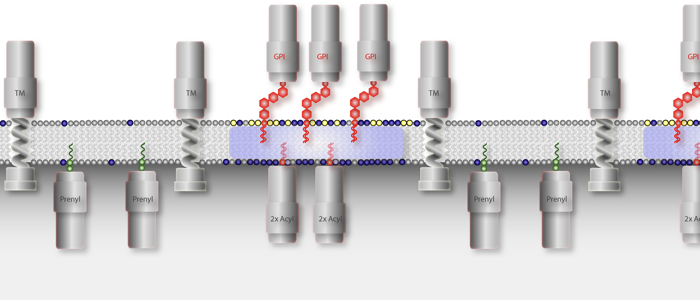
All eukaryotic cells express glycosylphosphatidylinositol (GPI)-anchored proteins. GPI-anchors are added post-transcriptionally by covalent linkage of a pre-assembled core anchor to the C-terminus of the protein. Whereas most eukaryotic cells express a varying number of GPI-anchored surface proteins in low abundance (mammalian cells express approx. 200 different proteins amounting to about 0.5 % of total cell surface proteins), the cell surface of African trypanosomes is dominated by a single GPI-anchored protein. We are utilizing this unusual abundance for the functional analysis of GPI-proteins. The first two complete structures of GPI-anchors were reported in 1988. One structure was that of rat Thy-1, the other one was from the major surface protein of the bloodstream form of Trypanosoma brucei, the variant surface glycoprotein (VSG). Since then it has become clear that all GPI-anchors are composed of the same core structure. The final composition of this anchor is modified by additions to the mannose moieties. The nature of these modifications and the attached lipids are governed by the species the protein is expressed in and by the protein itself. GPI-anchored proteins are attached to the cell surface via their lipid moieties and are therefore attached only to the outer leaflet of the plasma membrane. This gives GPI-anchored proteins unique characteristics compared to transmembrane proteins.
Negative sorting and extreme kinetics
Trypanosoma brucei internalizes all membrane through the flagellar pocket and via clathrin-coated vesicles. The endocytosed membrane along with fluid cargo is transported with bi-phasic kinetics via early endosomes to recycling endosomes. Strikingly, the Rab11-positive recycling endosomes are the place of VSG sorting: small clathrin-coated vesicles bud from extended, fenestrated structures, carrying the cargo via late endosomes to the single lysosome. These vesicles are devoid of VSG. Thus, by a negative sorting mechanism, i.e. the subtraction of membrane in the form of clathrin-vesicles, the GPI- anchored protein is passively (i.e by default) concentrated in recycling endosomes. Interestingly, the recycling endosomes that carry the increasingly concentrated VSG, eventually give rise to small, disk-shaped carriers that fuse with the flagellar pocket. These Rab11-positive exocytic carriers (EXCs) are abundantly found within the cell and were the first example for an endosome-derived structure fusing with the plasma membrane in any eukaryote. Trypanosomes recycle their entire VSG coat once every 7 minutes. Using time-resolved, quantitative colocalization analysis we have early-on been able to quantify the flow of membrane through the endosomal subcompartments (Engstler et al. 2004). From these analyses we can conclude that T. brucei reveals an exceptionally high rate of endocytic traffic. In fact, the flagellar pocket is endued with the highest endocytic capacity among all eukaryotes. Unravelling the molecular mechanisms underlying this extremely fast membrane traffic is still one of our prime goals, which is currently being tackled with novel techniques. Since the flagellar pocket area comprises just 5% of the total plasma membrane, it is difficult to envisage how an immediate re-endocytosis of just secreted (exocytosed) membrane can be avoided. Though our experiments strongly suggest that the flagellar pocket membrane is functionally polarized, we are still working on the proteins that might control this polarity.
Publications
Bartossek T, Jones NG, Schäfer C, Cvitković M, Glogger M, Mott HR, Kuper J, Brennich M, Carrington M, Smith A-S, Fenz S, Kisker C, Engstler M. (2017) Structural basis for the shielding function of the dynamic trypanosome VSG coat. Nature Microbiology 2, 1523–1532 doi.org/10.1038/s41564-017-0013-6
Hartel AJW, Glogger M, Jones NG, Abuillan W, Batram C, Hermann A, Fenz SF, Tanaka M, Engstler M (2016) N-glycosylation enables high lateral mobility of GPI-anchored proteins at a molecular crowding threshold. Nat Commun 7: 12870.
Hartel AJW, Glogger M, Guigas G, Jones NG, Fenz SF, Weiss M, Engstler M (2015) The molecular size of the extra-membrane domain influences the diffusion of the. Sci Rep 5: 10394.
Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P (2007) Hydrodynamic flow mediated protein sorting on the cell surface of trypanosomes. Cell 131: 505–515.
Overath P, Engstler M (2004) Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol 53: 735–744.
Engstler M, Thilo L, Weise F, Grunfelder CG, Schwarz H, Boshart M, Overath P (2004) Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J Cell Sci 117: 1105–1115.
Grunfelder CG, Engstler M, Weise F, Schwarz H, Stierhof Y-D, Morgan GW, Field MC, Overath P (2003) Endocytosis of a glycosylphosphatidylinositol-anchored protein via clathrin-coated vesicles, sorting by default in endosomes, and exocytosis via exocytic carriers. Mol Biol Cell 14: 2029–2040.
Grunfelder CG, Engstler M, Weise F, Schwarz H, Stierhof Y-D, Boshart M, Overath P (2002) Accumulation of a GPI-anchored protein at the cell surface requires sorting at multiple intracellular levels. Traffic 3: 547–559.