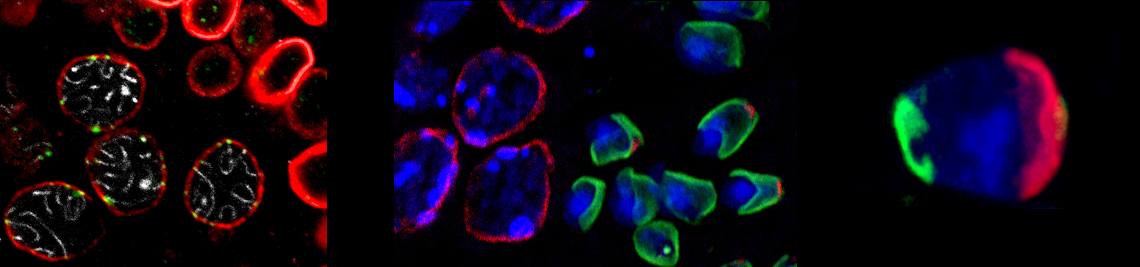
NUCLEAR STRUCTURE AND DYNAMICS
Nuclear structure and dynamics (Alsheimer lab)
Research focus
The defining characteristic of eukaryotes is the sequestration of the genetic material by the nuclear envelope (NE). The NE is composed of two nuclear membranes, nuclear pore complexes and the nuclear lamina, a proteinaceous meshwork that is in intimate contact with the nuclear side of the inner nuclear membrane. Besides its primary role in separating nuclear and cytoplasmic activities, the NE constitutes a most central component of intracellular architecture. It is vitally involved in many fundamental nuclear functions such as dynamic chromatin organization, transcription and replication. Several components of the NE were identified to function as key players in signalling pathways and recent studies evidenced that the NE has a pivotal role in nuclear migration, anchoring and positioning. Furthermore, the NE was demonstrated to be a most crucial determinant for maintaining nuclear morphology and shape and for general nuclear integrity. In addition to this, the NE turned out to serve as a kind of track for telomere driven chromosomal rearrangement during meiotic prophase I. Thus, from the current point of view the nuclear envelope represents more than just a simple barrier, rather it constitutes a multifunctional platform at the very center of fundamental cellular processes.
Research in our lab covers several aspects regarding the function of the nuclear envelope and its components during dynamic reorganization of nuclei. One main focus is set on the role of the nuclear envelope in the evolutionary highly conserved meiotic chromosome dynamics and its impact on genome haploidization. In a second core project we try to figure out the molecular mechanisms underlying the well-directed nuclear shaping during sperm head formation. Besides this, we are also interested in evolutionary aspects of meiosis, i.e. the evolution of the synaptonemal complex in metazoans.
Selected publications
Thoma, H., Grünewald, L., Braune, S., Pasch, E., and Alsheimer, M. (2023). SUN4 is a spermatid type II inner nuclear membrane protein that forms heteromeric assemblies with SUN3 and interacts with lamin B3. J. Cell Sci. 136, jcs.260155. doi.org/10.1242/jcs.260155
Pasch, E., Link, J., Beck, C., Scheuerle, S., and Alsheimer, M. (2015). The LINC complex component Sun4 plays a crucial role in sperm head formation and fertility. Biol. Open 4, 1792-1802. doi: 10.1242/bio.015768
Link, J., Leubner, M., Schmitt, J., Göb, E., Benavente, R., Jeang, K.-T., Xu, R., and Alsheimer, M. (2014). Analysis of meiosis in SUN1 deficient mice reveals a distinct role of SUN2 in mammalian meiotic LINC complex formation and function. PLoS Genet. 10, e1004099. doi: 10.1371/journal.pgen.1004099
Link, J., Jahn, D., Schmitt, J., Göb, E., Baar, J., Ortega, S., Benavente, R., and Alsheimer, M. (2013). The meiotic nuclear lamina regulates chromosome dynamics and promotes efficient homologous recombination in the mouse. PLoS Genet. 9, e1003261. doi: 10.1371/journal.pgen.1003261
Fraune, J.1, Alsheimer, M.1*, Volff, J.N., Busch, K., Fraune, S., Bosch, T.C.G., and Benavente, R.* (2012). Hydra meiosis reveals unexpected conservation of structural synaptonemal complex proteins across metazoans. Proc. Natl. Acad. Sci. USA 109, 16588-16593. doi: 10.1073/pnas.1206875109
Schramm, S., Fraune, J., Naumann, R., Hernandez-Hernandez, A., Höög, C., Cooke, H. J., Alsheimer, M.*, and Benavente, R.* (2011). A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination and fertility. PLoS Genet. 7, e1002088. doi: 10.1371/journal.pgen.1002088
Göb, E., Schmitt, J., Benavente, R., and Alsheimer, M. (2010). Mammalian sperm head formation involves different polarization of two novel LINC complexes. PLoS ONE 5, e12072. doi: 10.1371/journal.pone.0012072
Schmitt, J., Benavente, R., Hodzic, D., Höög, C., Stewart, C.L., and Alsheimer, M. (2007). Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc. Natl. Acad. Sci. USA 104, 7426-7431. doi: 10.1073/pnas.0609198104
LINC complexes and their relevance for nuclear remodelling, shaping and fertility
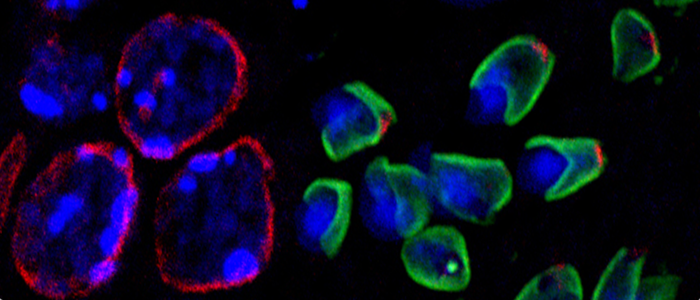
Regular sperm head formation implicitly depends on a quite extensive but well-directed nuclear remodelling that involves nuclear movement, compaction, polarization and reshaping form round to elongate. To date, the mechanisms how nuclei shape to form a functional sperm head are unknown. Recent studies have identified novel nuclear envelope bridging complexes, referred to as LINC complexes (linker of nucleoskeleton and cytoskeleton), that mediate a direct connection of nuclear structures with the cytoskeleton. LINC complexes are pivotal for nuclear migration, positioning and anchoring and for meiotic chromosome movements. Furthermore, they were shown to be essential for maintaining nuclear morphology and shape. Therefore, we propose that they could hold a key role in sperm head formation as well. Consistently, we found that nuclear elongation and reshaping closely correlates with formation and a conspicuous opposite polarization of two novel spermiogenesis-specific LINC complexes. Furthermore, we recently could identify Sun4 as key player in sperm-specific nuclear shaping. The aim of the current project is to further enlighten our still fragmentary knowledge about formation and composition of LINC complexes in spermatids and to determine the actual relevance of LINC complexes and their components for nuclear remodelling, shaping and fertility.
The nuclear envelope in meiotic chromosome dynamics
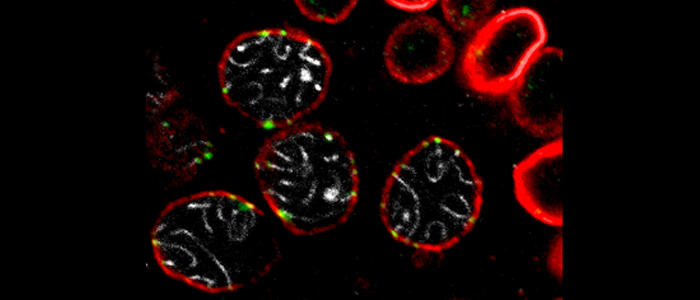
Germ cells are specialized cells which are of vital importance for eukaryotic life. They are the lynchpin of sexual reproduction and provide the enduring link between generations. Germ cells are designated to generate haploid cells that are committed to join with other gametes to create a new diploid organism. Therefore, during meiotic division cells have to recognize and unambiguously segregate the homologous chromosomes to reduce the genome.
It turned out that during meiotic prophase I the NE functions as a platform for the highly coordinated chromosomal movements which in turn are essential for pairing and recombination of the homologs. On entry into meiosis chromosomal ends firmly attach to nuclear envelope and start to move to congregate at one pole of the nucleus. Thereby chromosomes are trailed into close vicinity, a process that is supposed to further interhomolog interactions. Meiotic telomere attachment and their directed movements on the plane of the NE depend on a general reorganization of the NE. On the one hand, it concerns most central components of the inner nuclear membrane, which provide docking sites for telomeres. Attachment and movement of meiotic telomeres is mediated by the so-called LINC-complexes, which represent most central NE bridges that are used to transfer forces from the cytoplasm to the nucleus and, even more, across the NE to the nuclear interior.
At least in mammals, movement of telomeres within the NE requires a more general reorganization of the NE and in particular of the nuclear lamina. Mammalian meiotic cells lack three of the typically four somatic lamin isoforms, but instead express a unique short lamin, which is called lamin C2. We could show that this lamin, which can be considered as a kind of a “natural lamin deletion mutant”, holds a key function in chromosomal pairing and recombination and thus represents an essential determinant for fertility.
Evolution of the synaptonemal complex (SC)
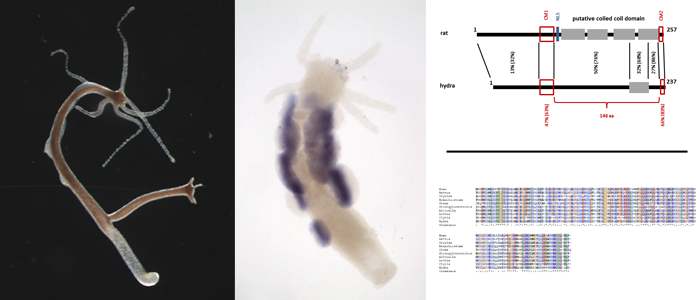
Meiosis is highly conserved in eukaryotic evolution. EM studies demonstrated the presence of synaptonemal complex (SC) like structures in fungi, plants and animals. However, the as yet identified proteins forming these structures in the different clades appear not to be genetically related. Even between the commonly used animal model organisms C. elegans, Drosophila and the mouse the main protein components of the SCs - even though they to some degree show structural similarity - seem to be not conserved on the amino acid level. This led to the conclusion that synaptonemal complexes may have evolved repeatedly during evolution.
Analyzing this issue in more detail, we in the past years could actually find that mammalian SC proteins are evolutionary highly related to that of basal metazoan organisms such as cnidarians and sponges. Thus, our results demonstrated that at least in metazoans the SC has a common origin, which includes the basic structural components as well as regulatory SC elements.