Tom Beneke
"Dass ich nicht mehr mit sauerm Schweiß Zu sagen brauche, was ich nicht weiß; Dass ich erkenne, was die Welt Im Innersten zusammenhält." Johann Wolfgang von Goethe, Faust
Tom Beneke
... is a molecular biologist with research experience in academia and industry. He completed his PhD at the University of Oxford where he developed novel CRISPR screening and gene editing approaches (LeishGEdit) to study the requirement of parasite motility throughout the Leishmania life cycle. This was followed by a PostDoc at the University of Oxford and a research position in the biotech industry. At OXGENE he employed large-scale CRISPR screens for target discovery projects. Tom was then awarded an EMBO and Marie Curie Fellowship to continue his research on Leishmania at the University of Würzburg and recently started his independent Junior Research Group at the Biocenter by acquiring additional funding from DRUID and the DFG.
Since 2024: Independent Junior Group Leader, Cell and Developmental Biology, Biocenter, University of Würzburg
Since 2023: Independent Marie-Sklodowska-Curie Postdoctoral Fellowship (hosted by Markus Engstler), Cell and Developmental Biology, Biocenter, University of Würzburg
2022 – 2023: Independent EMBO Postdoctoral Fellowship (hosted by Markus Engstler), Cell and Developmental Biology, Biocenter, University of Würzburg
2020 – 2022: CRISPR Screening Scientist/Senior Scientist, Oxford Genetics (OXGENE)
2019 – 2020: PostDoc in William James’ lab, Sir William Dunn School of Pathology, University of Oxford
2015 – 2019: PhD Student in Eva Gluenz’ lab, Sir William Dunn School of Pathology, University of Oxford
tom.beneke@uni-wuerzburg.de



Research synopsis
The Beneke lab is studying the protozoan parasite Leishmania which causes the neglected tropical disease leishmaniasis. This devastating disease causes approximately one million new cases and at least 20,000 deaths around the globe every year (WHO). Leishmania species are transmitted into humans by the bite of a sand fly. Once inside their mammalian host, they predominantly enter macrophages and can remain at the site of the bite (cutaneous leishmaniasis), disseminate throughout the skin (diffuse cutaneous/mucocutaneous leishmaniasis), or enter the bloodstream to establish visceral infections (visceral leishmaniasis). This is the difference between a mild illness or a deadly disease, and the fundamental mechanisms behind these different clinical manifestations are not fully understood.
The lab aims to dissect these mechanisms and test the hypothesis that different Leishmania species alter macrophage migration modes in distinct ways, to control dissemination through the dermis and across endothelial barriers. To determine the dynamics of parasite dissemination, the lab is comparing the spatiotemporal migration behaviour of Leishmania infected macrophages under in vitro and in vivo conditions. In addition, the Beneke lab is planning to set up high-throughput CRISPR screens in both, parasites and macrophages, to functionally dissect species-dependent mechanisms that enable Leishmania parasites to disseminate in their mammalian host.
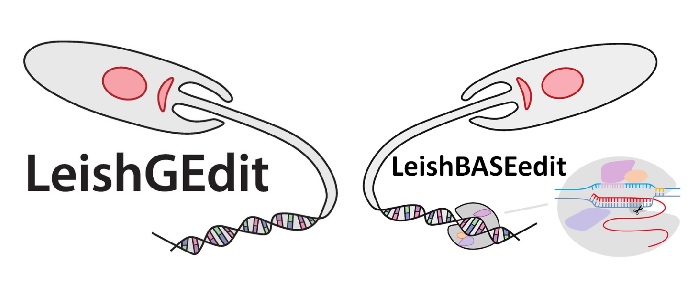
To pursue functional genetic screens in Leishmania, the lab has recently developed a new method (LeishBASEedit) which enables loss-of-function screening in Leishmania species via delivery of CRISPR cytosine base editing guide libraries. Through funding from DRUID and the DFG genome-wide loss-of-funtion libraries have been already produced and are now being utilized for a range of projects, including drug resistance and tissue tropism screens.
If you are interested in joining these efforts and want to become a member of the lab, please contact Tom via email (tom.beneke@uni-wuerzburg.de).
Current members of the Beneke lab are:
Nicole Herrmann May (PhD Student)

Jorge Adrián Arias del Angel (PostDoc)
Beatriz Cristina Dias De Oliveira (CAPES-Humboldt Research Fellowship Postdoc)

Sofie Fähr (Master Student)

Magdalena Priester (Master Student)

Ngoc Anh Cao (Master Student)

Mara Metz (Bachelor Student)

Nurzülal Erden (Master Student)
Hannah Hefter (Bachelor Student)
Alumni.... PostDocs:
- Fabian Link (DRUID funded)
- Tamara Sternlieb (Visiting PostDoc from Eva Gluenz lab)
Research Assistants:
- Konstantin Wawra
Master Projects:
- Annika Schmid
- Nicole Herrmann May
- Tulip Al-Hadawi
- Noah Wetterich
- Timothy Wuppermann
- Sarah Kießling
- Kristin Machleid
- Simon Schwind
- Ilkem Ekici
- Laura Finke
- Daana Morozova
Bachelor Projects:
- Judith Neye
- Hanna Sophie Wächter
- Annika Schmid
- Kristin Machleid
- Simon Schwind
- Simon Zorn
- Paul Schild
Publications
Pre-print Publications
Fochler S., Doran M. H., Beneke T., Smith J., Fort C., Walker B. J., Brown A., Gluenz E., Wheeler R. J. (2025). Axonemal dynein contributions to flagellar beat types and waveforms. BioRxiv. (Link)
Arias-del-Angel J., Link F., Herrmann May N., Ekici I., Wawra K., Schwind S., Zorn S., Haggarty J., Weidt S. K., Ritchie R., Barrett M. P., van Zandbergen G., Beneke T.. (2025). Genome-wide CRISPR base-editing screening defines drug response networks in Leishmania. BioRxiv. (Link)
Peer-reviewed Publications
Beneke T., Neish R., Catta-Preta C. M. C, Smith J., Valli J., McCoy C. J., Albuquerque-Wendt A., Mottram J. C., Gluenz E. (2025). Leishmania mexicana pathogenicity requires flagellar assembly but not motility. Virulence. (Link)
Doran M. J., Niu Q., Zeng J., Beneke T., Smith J., Ren P., Fochler S., Coscia A., Höög J. L., Meleppattu S., Lishko P. V., Wheeler R. J., Gluenz E., Zhang R., and Brown A. (2025). Evolutionary adaptations of doublet microtubules in trypanosomatid parasites. Science. 2025 Mar, Vol. 387, No. 6739. (Link)
Albuquerque-Wendt A., McCoy C. J., Neish R., Dobramysl U., Alagoez C., Beneke T., Cowley S. A., Crouch K., Wheeler R. J., Mottram J. C., Gluenz E. (2025). TransLeish: Identification of membrane transporters essential for survival of intracellular Leishmania parasites in a systematic gene deletion screen. Nature Communications. 2025 Jan 2;16(1):299. (Link)
Thiam R., Santana Ceballos M., Beneke T., Kuk N., Pasquier G., Crobu L., Jeffares D., Vergnes B., Barckmann B. and Sterkers Y. (2025). A novel Leishmania infantum reference strain for gene editing and the study of visceral leishmaniasis. PLoS One 20 (9), e0327390. (Link)
Herrmann May N., Cao A., Schmid A., Link F., Arias-del-Angel J., Meiser E. and Beneke T. (2024). Improved base editing and functional screening in Leishmania via co-expression of the AsCas12a ultra variant, a T7 RNA Polymerase, and a cytosine base editor. eLife 2024;13:RP97437. (Link)
Engstler M. and Beneke T. (2023). Gene editing and scalable functional genomic screening in Leishmania species using the CRISPR/Cas9 cytosine base editor toolbox LeishBASEedit. eLife 2023;12:e85605. (Link)
McCoy C. J., Paupelin-Vaucelle H., Gorilak P., Beneke T., Varga V. and Gluenz E. (2023). ULK4 and Fused/STK36 interact to mediate assembly of a motile flagellum. Mol Biol Cell, mbcE22060222. (Link)
Beneke T., Dobramysl U., Catta-Preta C. M. C., Mottram C. J., Gluenz E., Wheeler R. (2022). Genome sequence of Leishmania mexicana MNYC/BZ/62/M379 expressing Cas9 and T7 RNA polymerase. Wellcome Open Research, 7. (Link)
Sharma R., Avendaño Rangel F., Reis-Cunha J. L., Marques L. P., Figueira C. P., Borba P. B., Viana S. M., Beneke T., Bartholomeu D. C. and de Oliveira C. I. (2022). Targeted Deletion of Centrin in Leishmania braziliensis Using CRISPR-Cas9-Based Editing. Frontiers in Cellular and Infection Microbiology, 11. (Link)
Espada C. R., Quilles J. C., Albuquerque-Wendt A., Cruz M. C., Beneke T., Lorenzon L. B., Gluenz E., Cruz A. K. and Uliana S. R. B. (2021). Effective Genome Editing in Leishmania (Viannia) braziliensis Stably Expressing Cas9 and T7 RNA Polymerase. Frontiers in Cellular and Infection Microbiology, 11. (Link)
Andersson M., [...], Beneke T. et al. (2020). SARS-CoV-2 RNA detected in blood samples from patients with COVID-19 is not associated with infectious virus. Wellcome Open Research Oct 12;5:181. (authors in alphabetically order). (Link)
Wang Z.*, Beneke T.*, Gluenz E. and Wheeler R. J. (2020). The single flagellum of Leishmania has a fixed polarisation of its asymmetric beat. Journal of Cell Science 133: jcs246637. (Link)
Beneke T., Banecki K., Fochler S. and Gluenz E. (2020). LAX28 is required for assembly of the inner dynein arm l1 and tether/tether head complex in the Leishmania flagellum. Journal of Cell Science 133: jcs239855. (Link)
Beneke T. and Gluenz E. (2020). Bar-seq strategies for the LeishGEdit toolbox. Molecular and Biochemical Parasitology 239, 111295. (Link)
Beneke T., Demay F., Hookway E., Ashman N., Jeffery H., Smith J., Valli J., Becvar T., Myskova J., Lestinova T., Shafiq S., Sadlova J., Volf P., Wheeler R. J., and Gluenz E. (2019). Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog 15, e1007828. (Link)
Schädeli D., Serricchio M., Ben Hamidane H., Loffreda A., Hemphill A., Beneke T., Gluenz E., Graumann J. and Bütikofer P. (2019). Cardiolipin depletion-induced changes in the Trypanosoma brucei proteome. FASEB J Dec;33(12):13161-13175. (Link)
Costa F. C., Francisco A. F., Jayawardhana S., Calderano S. G., Lewis M. D., Olmo F., Beneke T., Gluenz E., Sunter J., Dean S., Kelly J. M. and Taylor M. (2018). Expanding the toolbox for Trypanosoma cruzi: A parasite line incorporating a bioluminescence-fluorescence dual reporter and streamlined CRISPR/Cas9 functionality for rapid in vivo localisation and phenotyping. PLoS Neg Trop Dis 12, e0006388. (Link)
Martel D., Beneke T., Gluenz E., Spaeth G. and Rachidi N. (2017). Characterisation of Casein kinase 1.1 in Leishmania donovani using the CRISPR Cas 9 toolkit. BioMed Res International 2017:4635605. (Link)
Beneke T.*, Madden R.*, Makin L., Valli J., Sunter J. and Gluenz E. (2017). A CRISPR Cas9 high-throughput genome editing toolkit for kinetoplastids. R Soc Open Sci 4, 170095. (Link)
Book chapters
Beneke T., Demay F., Wheeler R. J. and Gluenz E. (2020). Isolation of Leishmania promastigote flagella. Methods in Molecular Biology Trypanosomatids 2116:485-495. (Link)
Beneke T. and Gluenz E. (2019). LeishGEdit: a method for rapid gene knockout and tagging using CRISPR/Cas9. Methods in Molecular Biology Trypanosomatids 1971:189-210. (Link)