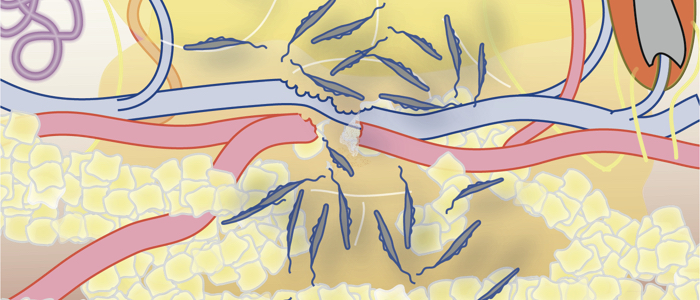
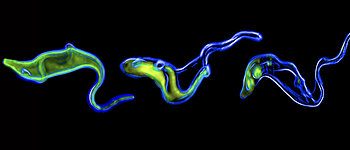
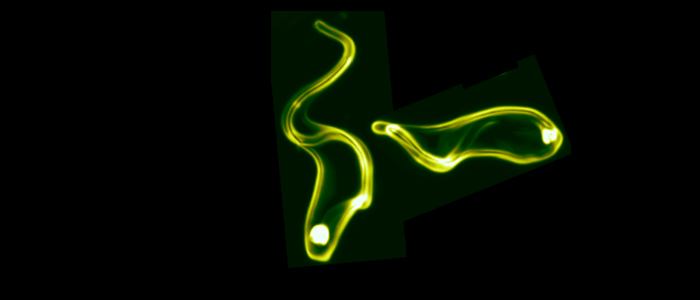
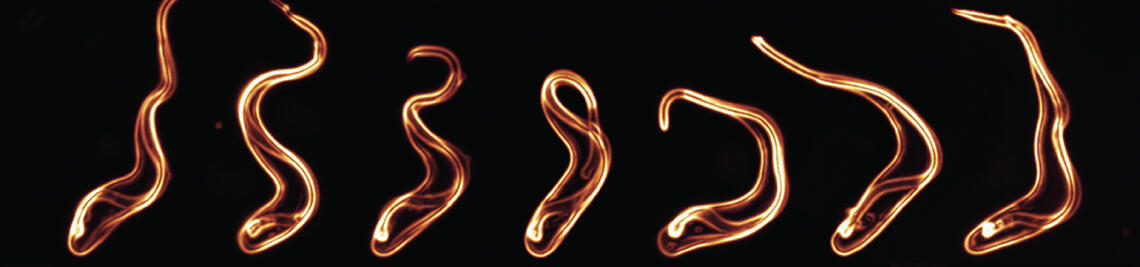Subcellular dynamics in unicellular parasites. Müller T, Krüger T, Engstler M. Trends Parasitol. 2025 Mar;41(3):222-234. doi: 10.1016/j.pt.2025.01.007. Epub 2025 Feb 10. PMID: 39933989.
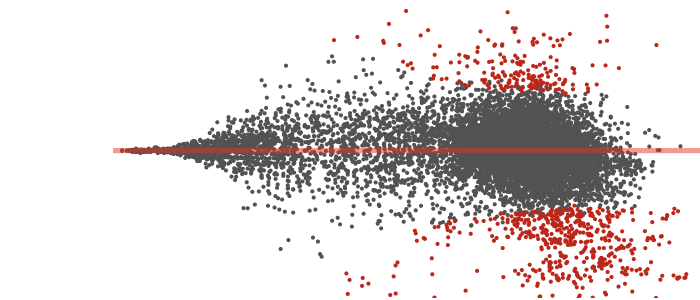
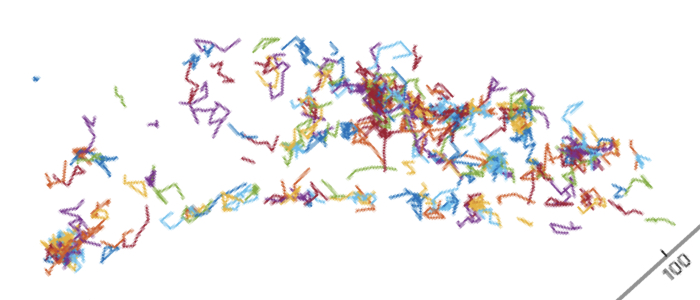
ThirdPeak is a flexible tool designed for the robust analysis of two- and three-dimensional tracking data. Müller T, Meiser E, Engstler M. Commun Biol. 2024 Dec 20;7(1):1683. doi: 10.1038/s42003-024-07378-w. PMID: 39702822; PMCID: PMC11659616.
The actomyosin system is essential for the integrity of the endosomal system in bloodstream form Trypanosoma brucei. Link F, Jung S, Malzer X, Zierhut F, Konle A, Borges A, Batters C, Weiland M, Poellmann M, Nguyen AB, Kullmann J, Veigel C, Engstler M, Morriswood B. Elife. 2024 Nov 21;13:RP96953. doi: 10.7554/eLife.96953. PMID: 39570285; PMCID: PMC11581428.
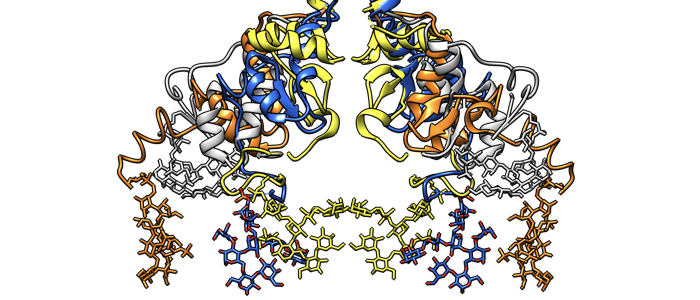
The release of host-derived antibodies bound to the variant surface glycoprotein (VSG) of Trypanosoma brucei cannot be explained by pH-dependent conformational changes of the VSG dimer. Eirich P, Nesterov P, Shityakov S, Skorb EV, Sander B, Broscheit J, Dandekar T, Jones NG, Engstler M. Open Res Eur. 2024 Apr 24;4:87. doi: 10.12688/openreseurope.16783.1. PMID: 38903703; PMCID: PMC11187536.
Continuous endosomes form functional subdomains and orchestrate rapid membrane trafficking in trypanosomes. Link F, Borges A, Karo O, Jungblut M, Müller T, Meyer-Natus E, Krüger T, Sachs S, Jones NG, Morphew M, Sauer M, Stigloher C, McIntosh JR, Engstler M. Elife. 2024 Apr 15;12:RP91194. doi: 10.7554/eLife.91194. PMID: 38619530; PMCID: PMC11018342.
Vector-borne Trypanosoma brucei parasites develop in artificial human skin and persist as skin tissue forms. Reuter C, Hauf L, Imdahl F, Sen R, Vafadarnejad E, Fey P, Finger T, Jones NG, Walles H, Barquist L, Saliba AE, Groeber-Becker F, Engstler M. Nat Commun. 2023 Nov 23;14(1):7660. doi: 10.1038/s41467-023-43437-2. PMID: 37996412; PMCID: PMC10667367.
Gene editing and scalable functional genomic screening in Leishmania species using the CRISPR/Cas9 cytosine base editor toolbox LeishBASEedit. Engstler M, Beneke T. Elife. 2023 May 24;12:e85605. doi: 10.7554/eLife.85605. PMID: 37222701; PMCID: PMC10208639.
The Intracellular Amastigote of Trypanosoma cruzi Maintains an Actively Beating Flagellum. Won MM, Krüger T, Engstler M, Burleigh BA. mBio. 2023 Apr 25;14(2):e0355622. doi: 10.1128/mbio.03556-22. Epub 2023 Feb 22. PMID: 36840555; PMCID: PMC10128032.
Nanobody-mediated macromolecular crowding induces membrane fission and remodeling in the African trypanosome.
Hempelmann A, Hartleb L, van Straaten M, Hashemi H, Zeelen JP, Bongers K, Papavasiliou FN, Engstler M, Stebbins CE, Jones NG.
Cell Rep. 2021 Nov 2;37(5):109923. doi: 10.1016/j.celrep.2021.109923. PMID: 34731611
Unexpected plasticity in the life cycle of Trypanosoma brucei.
Schuster S, Lisack J, Subota I, Zimmermann H, Reuter C, Mueller T, Morriswood B, Engstler M.
Elife. 2021 Aug 6;10:e66028. doi: 10.7554/eLife.66028. PMID: 34355698
Single-cell motile behaviour of [Formula: see text] in thin-layered fluid collectives.
Krüger T, Maus K, Kreß V, Meyer-Natus E, Engstler M.
Eur Phys J E Soft Matter. 2021 Mar 23;44(3):37. doi: 10.1140/epje/s10189-021-00052-7. PMID: 33755816